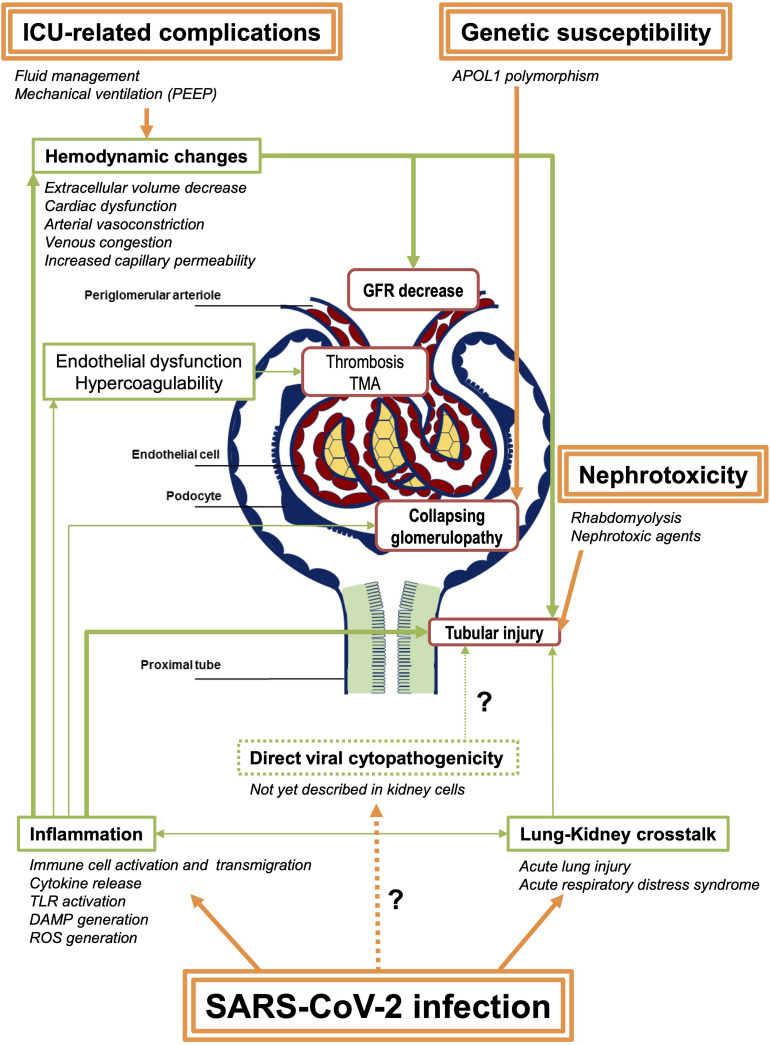
FIGURE 1.
Summary of the potential pathophysiological mechanisms of SARS-CoV-2–associated AKI. SARS-CoV-2 infection induces direct pulmonary injury that might lead to systemic inflammation. Hemodynamic changes are also frequent in patients admitted in ICU, due to the infection and its complication, as well as to medical interventions. These modifications result in GFR decrease and thus prerenal azotemia, potentially leading to acute ischemic tubular injury. Other factors including inflammation itself and tubular toxicity due to nephrotoxic agents (antibiotics, antiviral drugs, etc.) contribute to acute tubular injury in these patients. Few cases of TMA and renal vascular thrombosis have also been reported, raising the hypothesis of endothelial dysfunction and systemic hypercoagulability in the most severe patients. Collapsing glomerulopathy is a specific feature of SARS-CoV-2–related AKI, also called COVAN (COVID-associated nephropathy) in reference to HIVAN (HIV-associated nephropathy), as they probably share common mechanisms, including the strong association with APOL1 genetic variants. Finally, following the report of the autopsy series from Puelles et al. (2020), a direct tubular or glomerular viral invasion has not yet been confirmed in other reports. Consequently, this mechanism remains controversial. Arrows in bold represent the proposed major mechanisms. PEEP, positive end-expiratory pressure; GFR, glomerular filtration rate; TMA, thrombotic microangiopathy; TLR, Toll-like receptors; DAMP, damage-associated molecular patterns; ROS, reactive oxygen species.