Figure 1.
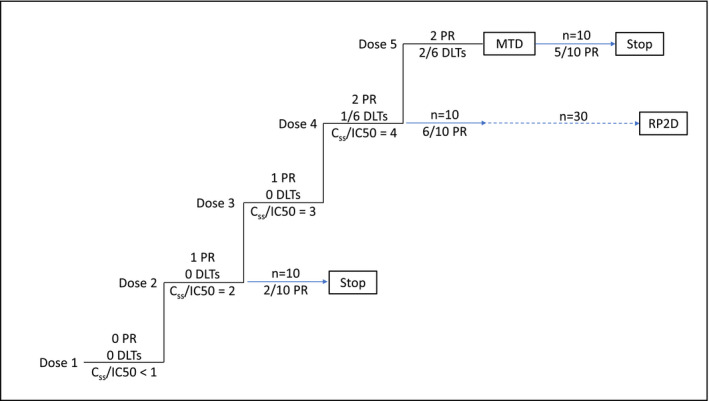
Potency‐guided first‐in‐human trial design, including theoretical outcomes. Dose expansion is initiated at dose level 2 when the steady‐state concentration (Css) value is 2‐fold greater than the half‐maximal inhibitory concentration (IC50) with no DLTs and 1 PR. Dose expansion is also initiated at dose levels 4 and 5 (the maximum tolerated dose (MTD)). Comparison of the first 10 patients in the 3 expansion cohorts suggests dose level 4 is most promising and further enrollment is limited to dose level 4. Dose levels 3 is not selected for expansion as exposure is overlapping, due to pharmacokinetic variability, with adjacent dose levels. Blue arrows represent enrollment into dose expansion cohorts. DLT, dose limiting toxicity; PR, partial response; RP2D, recommended phase 2 dose.
