Figure 1.
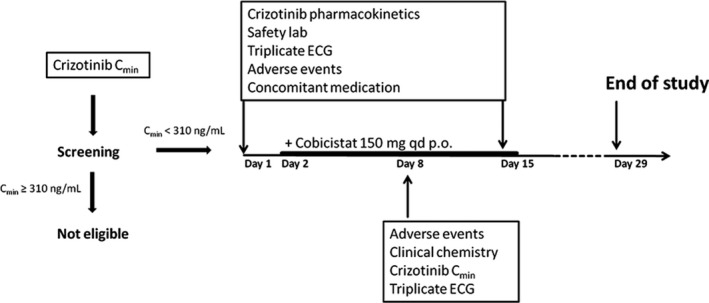
Crizotinib trough concentrations (Cmin,ss) were measured at the start of the trial. Patients with Cmin,ss < 310 ng/mL were eligible for cobicistat treatment. On day 1 and day 15 we collected pharmacokinetic (PK) samples over the 12‐hour dosing interval, safety laboratory, and triplicate echocardiogram (ECG). Oral treatment with 150 mg cobicistat q.d. was started on day 2. A safety visit was scheduled on day 8 and day 29.
