Abstract
Pharmacogenetic (PGx) testing is a tool to identify patients at a higher risk of adverse events or treatment failure. The concern for unwanted side effects can limit medication adherence, particularly in children and adolescents. We conducted a pragmatic study to evaluate the acceptability and feasibility and gather pilot data on the utility of PGx testing in a child and adolescent psychiatry clinic. Both physicians and families participated in the study and answered pre‐survey and post‐survey questionnaires to examine their attitudes toward PGx testing. Patients were randomized into implementation (N = 25) and control groups (N = 24) and underwent PGx testing at the beginning or end of the study, respectively. Clinical consult notes with genotype‐guided recommendations were provided to physicians for their consideration in clinical decisions. Patient‐reported symptom severity and antidepressant‐related side effects were assessed at baseline and for 12 weeks. Both participating physicians and families agreed that PGx testing is a useful tool to improve medication selection. The time from sample collection to having PGx test results was ~ 10 days and 15 days to having consult notes available, which may have impaired test utility in clinical decision making. There were no differences in any clinical end point between the implementation and control arms; however, there were higher antidepressant side effect scores for CYP2D6 poor and intermediate metabolizers after the eighth week of treatment. Our findings revealed benefits and pitfalls with the use of PGx testing in the real‐world clinical setting, which may inform the methodology of a larger trial focused on outcomes.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Previous studies have demonstrated that differences in CYP2D6 and CYP2C19 explains variability in drug response of psychiatric medications in adults. There is a need to assess the acceptability, feasibility, and clinical utility of psychiatric medications among the pediatric population in the real clinical setting.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ This study aimed to address whether pharmacogenetic (PGx) testing is accepted among parents and physicians, is feasible in a real world clinical setting, and is useful to choose optimal medications to treat depression in the pediatric population.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ This study found that PGx testing is feasible and well‐accepted among physicians and families of children with depression and anxiety and has the potential to identify patients at higher risk of experiencing side effects after 8 weeks of treatment. Our study also identified challenges for PGx testing implementation for treatment of pediatric psychiatric disorders in the real‐world clinical setting.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ Our findings informed the methodology for a large randomized clinical trial investigating PGx testing in both children and adults with psychiatric disorders.
Approximately one in every six children and adolescents suffers from a psychiatric illness, the most common being anxiety and depression. 1 , 2 Although psychosocial interventions are effective, pharmacological therapy is needed in more severe cases. The use of selective serotonin reuptake inhibitors (SSRIs) and serotonin‐norepinephrine reuptake inhibitors either alone 3 or in combination with cognitive behavioral therapy 4 is beneficial for the treatment of depression and anxiety disorders in children and adolescents. Nevertheless, these medications also carry a substantial potential side effect burden.
Optimization of antidepressant therapy in children can be challenging given the potential for adverse events, particularly in the first few days of treatment, 5 consequently impacting treatment adherence. In fact, experiencing physical adverse events related to antidepressants is not uncommon in the pediatric population and may lead to visits to the emergency department. 6 Adherence may also be impacted by parents’ perceptions that the treatment may cause more harm than benefit. 7 Thus, identifying a medication that is most likely to be effective and/or has a lower risk of potential side effects in advance of treatment initiation could lead to an improvement in outcomes.
One potential way to optimize medication choice and dosage is the use of pharmacogenetics (PGx), which involves testing specific variants in genes encoding for drug metabolizing enzymes (pharmacokinetics) or target proteins (pharmacodynamics). 8 CYP2D6 and CYP2C19 are the primary enzymes responsible for the metabolism of SSRIs, one of the most commonly used classes of antidepressants in adults and children. Polymorphisms in the genes that encode for CYP2D6 and CYP2C19 may contribute to interindividual differences in the pharmacokinetics of SSRIs. 9 The CYP2D6 and CYP2C19 genes are highly polymorphic, conferring normal metabolizer (NM), intermediate metabolizer (IM), poor metabolizer (PM), and rapid or ultra‐rapid metabolizer phenotypes (RM and UM, respectively). Although the sample size in most PGx association studies conducted in the pediatric population is limited, 10 several studies have shown an association between CYP2D6 and CYP2C19 genotypes and antidepressants response. 11 , 12 , 13 , 14 Nevertheless, there is still much work to be done regarding assessing the acceptability, feasibility, and utility of PGx testing in children with psychiatric disorders, particularly in the outpatient setting.
This study was a prospective, randomized, pragmatic clinical trial comparing antidepressant therapy in children and adolescents using a genotype‐guided approach vs. the standard of care. The primary aim was to assess the acceptability and feasibility of PGx testing in a child psychiatry clinic. Our primary outcomes were: (i) attitudes of parents and physicians towards PGx testing (acceptability), and (ii) ease of and barriers to the implementation of PGx testing in a child psychiatry clinic (feasibility). As secondary outcomes, we evaluated the utility of PGx testing by measuring clinical end points related to medication response and psychiatric adverse events in the PGx implementation vs. the control arm (usual treatment).
METHODS
Study setting and design
This study was conducted at the University of Florida (UF) Health outpatient Pediatric Psychiatry Clinic and was approved by the institutional review board and registered on ClinicalTrials.gov (NCT02855580). The clinic provides evaluation and treatment for children and adolescents up to age 20 years. The study procedure is summarized in Figure 1 .
Figure 1.
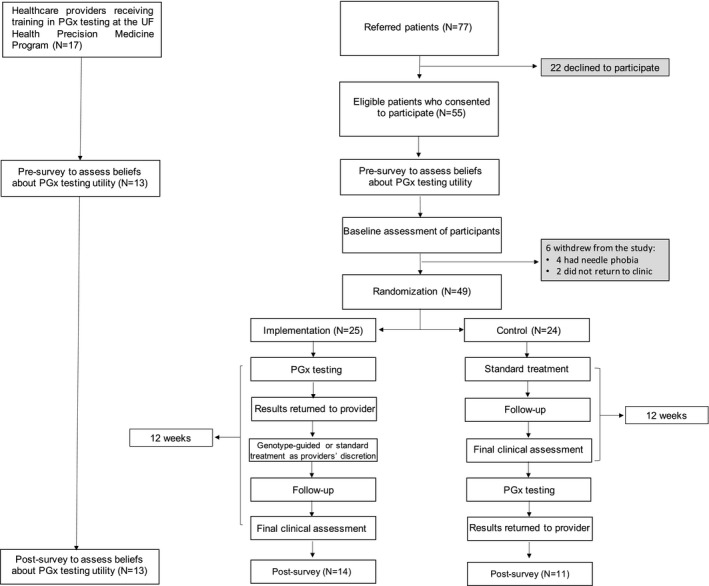
Diagram of the study procedure. PGx, pharmacogenetics; UF, University of Florida.
Healthcare providers
Physicians in the UF Health Pediatric Psychiatry Clinic (faculty and fellows) were consented to participate in the study to evaluate the acceptability of PGx testing for their patients and use of PGx results in prescribing decisions. A pharmacist from the UF Health Precision Medicine Program (PMP) conducted an in‐person educational session on psychiatric PGx using written and oral educational materials and case discussions. The training incorporated patient cases and included discussion of topics, such as ordering a PGx test at UF, genes included in the testing, interpretation of results, understanding the association between PGx profiles and treatment outcomes, and applying PGx results to prescribing decisions for antidepressant therapy.
Patients
Participating physicians referred eligible patients to the study but did not consent them to avoid selection bias. Nonparticipating physicians also referred patients to the study. Families were consented by study investigators; parents provided informed parental permission, whereas children of age 12 years and under provided assent and adolescents (ages 13–18 years) provided consent. Patients under age 20 years in whom initiation or dosage change of an SSRI was being considered for treatment for a depressive, anxiety, or obsessive‐compulsive disorder were eligible for participation. Children or adolescents with primary diagnoses of autism or psychotic disorders or at high risk of suicide were excluded to avoid a delay in starting or changing therapy and potential harm in these populations. Patients were assessed for baseline demographics (age, sex, race, and ethnicity) and clinical characteristics (psychiatric diagnosis, medication use, and symptom scores), and randomized by the investigators 1:1 to the PGx implementation or the control group (usual treatment). All participants received CYP2D6 and CYP2C19 genotyping independent of their study arm.
Assessment of PGx testing acceptability
Attitudes toward PGx testing in study participants (physicians, parents, and adolescents older than age 12 years) were assessed at week 0 (pre‐survey) and week 15 (post‐survey). The surveys asked participants to rate their responses on a scale of 1 (strongly disagree) to 5 (strongly agree). The family survey assessed views regarding the use of antidepressants, willingness to undergo genetic testing and/or delay treatment until receiving test results, perceived risks (e.g., insurance coverage and discrimination based on PGx test results), and willingness to pay for PGx testing. Physician surveys assessed knowledge about CYP2D6 and CYP2C19 variability and its impact on antidepressant therapy, confidence in using PGx testing for their patients, views about integrating PGx to their clinical practice, and the benefits of PGx testing to choose medications for their patients.
Assessment of PGx testing feasibility
DNA from the implementation group was sent for PGx testing as soon as collected; in the control group, DNA was stored, and testing occurred after week 12 of the study. DNA was collected from blood samples, as at the time of the study, this was the only validated collection method in the UF Health Pathology Laboratory, which is accredited by the College of American Pathologists and certified by the Clinical Laboratory Improvement Amendments of 1988 (CLIA).
CYP2D6 genotype was determined using Luminex xTAG CYP2D6 Kit version 3 (Luminex, Austin, TX), as previously described. 15 Each allele was assigned an activity value to obtain an activity score. Translation from activity score to CYP2D6 phenotype followed recommendations from the Clinical Pharmacogenomics Implementation Consortium (CPIC) guidelines available at the time the study was conducted, 16 however, we acknowledge updated translation is available. 17 Drug‐induced phenoconversion of CYP2D6 was determined in individuals taking a strong CYP2D6 inhibitor (e.g., bupropion), and were considered as PMs in the analyses, as previously described. 15
CYP2C19 genotypes were determined using the GenMark Dx (Carlsbad, CA) platform, as previously described. 15 Individuals with two no‐function alleles (e.g., *2 through *6) were assigned the PM phenotype, those with one no‐function allele were assigned the IM phenotype, and those with one normal (*1) and one increased function allele (*17) or two were assigned the RM (*1/*17) or UM (*17/*17) phenotype, respectively.
Once PGx test results of both CYP2D6 and CYP2C19 were available for individuals in the implementation group, a UF Health PMP pharmacist provided a clinical consult note that included an interpretation of the results and genotype‐guided recommendations (see Table S1 ). The note was routed via message to the ordering provider in the Epic Electronic Health Record (EHR). In addition to the consult notes, Best Practice Advisory alerts were introduced about halfway through the study. In the presence of genotype results in the EHR, an active alert would fire if the provider ordered a medication that was not recommended based on the individual’s genotype (e.g., CYP2C19*17/*17 or UM and escitalopram). The healthcare provider was able to order a change in medication based on phenotype information at his/her discretion. The number of physicians who acknowledged the PGx test results for dosing recommendations and/or referred to the results in progress notes was recorded.
Assessment of PGx testing utility
The number of times that a medication was concordant (or consistent) with an actionable phenotype was determined. Concordance was defined as starting or continuing an SSRI or switching to a different one not metabolized by the relevant enzyme (CYP2D6 or CYP2C19) or appropriately increasing/decreasing the dose of the SSRI based on actionable phenotype.
Actionable phenotypes were defined in two fashions: as either standard or extended. The standard definition considers UM/RM or PM as actionable phenotypes for either CYP2D6 and/or CYP2C19 consistent with the CPIC guidelines. 16 The extended definition included IM as an actionable phenotype based on evidence suggesting that reduced activity may lead to increased effective drug doses and thus affect treatment outcomes. 18 As the CPIC guidelines do not define these phenotypes as actionable, the extended definition was not examined with regard to medication concordance; this definition was only used in analyses examining participant outcomes by phenotype. Actionable phenotypes were assigned for both CYP2C19 and CYP2D6 separately.
A secondary outcome of this study was to evaluate the utility of PGx testing by measuring clinical end points to gather preliminary data to inform a larger trial of the efficacy of genotype‐guided antidepressant therapy. Participants (for children under age 12 years, together with their parents) completed surveys to assess psychiatric symptoms, severity, and side effects of the medications in each study arm at weeks 0, 1, 2, 4, 8, and 12. Global measures of impairment were assessed using the Columbia Impairment Scale (CIS), this questionnaire targets performance of the individual in his/her school or job, interpersonal relationships, use of free time, and psychopathological domains. 19 The presence of side‐effects was assessed using the Antidepressant Side‐Effect Checklist (ASEC) that includes physical symptoms commonly experienced with the use of antidepressants. 20 We did not assess medication safety, such as rashes. Depressive symptoms were measured using the Children’s Depression Inventory (CDI), which is a self‐report scale designed for young individuals from 7–17 years of age. 21 Obsessive compulsive and anxiety symptoms were self‐reported with the Obsessive Compulsive Inventory‐Revised (OCI‐R) 22 and the Screen for Child Anxiety Related Emotional Disorders (SCARED), 23 respectively. Overall, higher scores were indicative of worse disease severity (CDI, OCI‐R, SCARED, and CIS) and higher number of side effects (ASEC).
Data analysis
Baseline characteristics of participants in the control vs. the implementation group were compared using a χ2 test or t‐test, as appropriate. If participants were missing data, their data were not included in the relevant analyses. The acceptability of PGx testing was analyzed by comparing the proportion of participants who agreed with each item in the pre‐survey vs. the post‐survey using a χ2 test. Although the study was not powered to detect meaningful clinical changes, analyses examining differences in patient outcomes using the CIS and ASEC between study arms were also conducted to assess the utility of PGx testing. Finally, trajectories of the clinical end points across study arms and phenotypes were explored as post hoc analyses.
For the clinical end points (utility) analyses, we used a mixed linear model that relied on maximum likelihood estimation and variance components structure to estimate fixed and random effects. This model tested longitudinal effects of study arm and genotype differences, including potential group‐by‐time and phenotype‐by‐time interactions, on each of the clinical end points. Phenotypes were collapsed into three main categories to facilitate the analysis: PM/IM, NM, and RM/UM. Two hierarchical models were tested that incorporated the fixed effects of randomization groups (implementation vs. control) or phenotypes (PM/IM vs. NM vs. RM/UM), as well as fixed effects of group‐by‐linear time and group‐by‐quadratic time interactions. Analyses evaluating −2 log likelihood statistic, Akaike’s Information Criterion, and Schwarz’s Bayesian Criterion fit statistics against the null model were conducted to determine the best fitting model; χ2 analyses were run to determine if there were significant changes in −2 log likelihood. All statistical results for exploratory analyses were reported without correction for multiple testing.
RESULTS
Study participants
A total of 17 physicians participated in the study and referred the majority of patients (N = 40), whereas nonparticipating physicians referred the remaining study participants. A total of 77 eligible families were invited to participate, of which 55 consented. Two patients declined to participate further and four withdrew because of the need for venipuncture for sample collection (Figure 1 ). The ages of the patients ranged from 8 to 20 years. Forty‐nine patients were randomized into the implementation (N = 25) or the control (N = 24) groups. Of these 49 participants, 38 (77%) were on a medication at baseline and were considering a medication change, primarily due to side effects (63%, N = 24), whereas 11 (23%) were considering starting a medication. The baseline characteristics of the participants are available in Table 1 . There were no differences in baseline CIS or ASEC scores between those on medication at baseline and those not on a medication, after controlling for age and sex.
Table 1.
Baseline characteristics of patients
| Characteristic | Control (N = 24) | Implementation (N = 25) | P value |
|---|---|---|---|
| Age, mean ± SD | 14.8 ± 3.2 years | 14.5 ± 3.6 years | Pr(|T|> |t|) = 0.78 |
| Sex, n (%) | |||
| Female | 12 (50.0) | 19 (76.0) | 0.06 |
| Race, n (%) | 1.00 | ||
| White | 21 (87.5) | 21 (84.0) | |
| Other a | 3 (12.5) | 4 (16.0) | |
| Ethnicity, n (%) | 1.00 | ||
| Hispanic or Latino | 3 (12.5) | 4 (16.0) | |
| Non‐Hispanic or Latino | 21 (87.5) | 21 (84.0) | |
| Primary diagnosis, n (%) | 0.49 | ||
| ADHD | 1 (4.2) | 0 (0.0) | |
| Anxiety | 6 (25.0) | 8 (32.0) | |
| Depression | 11 (45.8) | 14 (56.0) | |
| OCD | 6 (25.0) | 3 (12.0) | |
| Medications, n (%) | 0.74 | ||
| SSRIs | |||
| Citalopram | 1 (4.2) | 0 (0.0) | |
| Escitalopram | 5 (20.8) | 4 (16.0) | |
| Fluoxetine | 4 (16.7) | 4 (16.0) | |
| Fluvoxamine | 3 (8.3) | 1 (4.0) | |
| Sertraline | 7 (29.2) | 6 (24.0) | |
| No SSRI/other antidepressant | 2 (8.3) | 5 (20.0) | |
| Non‐SSRIs antidepressants | 1 (4.2) | 0 (0.0) | |
| Other antidepressants b | 0.88 | ||
| Bupropion | 2 (8.3) | 1 (4.0) | |
| Doxepin | 1 (4.2) | 1 (4.0) | |
| Quetiapine | 1 (4.2) | 0 (0.0) | |
| Trazodone | 1 (4.2) | 1 (4.0) | |
| Other psychiatric medications c | |||
| Amphetamine | 0 (0.0) | 1 (4.0) | 1.00 |
| Aripiprazole | 5 (20.8) | 0 (0.0) | 0.02 |
| Atomoxetine | 2 (8.3) | 1 (4.0) | 0.61 |
| Benztropine | 2 (8.3) | 0 (0.0) | 0.23 |
| Buspirone | 1 (4.2) | 1 (4.0) | 1.00 |
| Clonidine | 1 (4.2) | 0 (0.0) | 1.00 |
| Dexmethylphenidate | 1 (4.2) | 0 (0.0) | 1.00 |
| Diazepam | 1 (4.2) | 0 (0.0) | 1.00 |
| Guanfacine | 2 (8.3) | 3 (12.0) | 1.00 |
| Hydroxyzine | 1 (4.2) | 0 (0.0) | 1.00 |
| Lamotrigine | 0 (0.0) | 1 (4.0) | 1.00 |
| Lisdexamfetamine | 0 (0.0) | 2 (8.0) | 0.23 |
| Methylphenidate | 5 (20.8) | 1 (4.0) | 0.09 |
| Naltrexone | 1 (4.2) | 0 (0.0) | 1.00 |
| Olanzapine | 1 (4.2) | 0 (0.0) | 1.00 |
| Perphenazine | 1 (4.2) | 0 (0.0) | 1.00 |
| Propanolol | 1 (4.2) | 1 (4.0) | 1.00 |
| Risperidone | 3 (12.5) | 2 (8.0) | 1.00 |
| Topiramate | 1 (4.2) | 1 (4.0) | 1.00 |
| Medication plan, n (%) | 0.74 | ||
| Start medication | 5 (20.8) | 7 (28.0) | |
| Switch medication | 19 (79.2) | 18 (72.0) | |
| Symptom scores, mean ± SD | |||
| ASEC score | 12.6 ± 9.3 | 15.4 ± 2.6 | Pr(|T|> |t|) = 0.392 |
| CDI score | 45.0 ± 10.2 | 44.7 ± 13.8 | Pr(|T|> |t|) = 0.927 |
| CIS score | 22.1 ± 9.9 | 22.6 ± 7.5 | Pr(|T|> |t|) = 0.825 |
| SCARED score | 32.6 ± 16.3 | 35.0 ± 18.8 | Pr(|T|> |t|) = 0.622 |
| OCI‐R | 13.1 ± 7.4 | 12.9 ± 9.1 | Pr(|T|> |t|) = 0.945 |
ADHD, attention deficit hyperactivity disorder; ASEC, Antidepressant Side‐Effect Checklist; CDI, Children’s Depression Inventory; CIS, Columbia Impairment Scale; OCD, obsessive‐compulsive disorder; OCI‐R, Obsessive Compulsive Inventory‐Revised; SCARED, Screen for Child Anxiety Related Emotional Disorders; SSRI, selective serotonin reuptake inhibitor.
Other includes African Americans and American Indian or Alaskan.
Other non‐SSRIs antidepressants that were used alone or in combination with SSRIs.
One individual may have more than one medication and each medication can be used either alone or in combination with SSRIs and/or other antidepressants.
Acceptability of PGx testing
Healthcare providers
Thirteen of the 17 (76%) providers completed the surveys (Table 2 ). More than 90% of the physicians felt confident using PGx testing results at the post‐survey in comparison with only 46.2% at the beginning of the study. The number of physicians who endorsed PGx testing as a tool that fits in their way of managing their patients doubled from pre‐survey to post‐survey. Moreover, 100% of the physicians reported that using genetic data to guide therapeutic choices improved their ability to select medications at the end of the study (post‐survey) compared with 61.5% at the pre‐survey. Physicians were willing to wait an average of 2 weeks for PGx testing results prior to starting patients on a medication.
Table 2.
Attitudes about PGx testing among physicians
| Survey question |
Pre‐survey: “agree” or “strongly agree” N = 12 |
Post‐survey: “agree” or “strongly agree” N = 13 |
|---|---|---|
| Understand the role of CYP2D6 and CYP2C19 genotype testing in prescribing medications | 12 (92.3%) | 13 (100%) |
| In favor of adding genotype ordering process | 10 (76.9%) | 13 (100%) |
| Confident in ability to use results of genotype testing | 6 (46.2%) | 12 (92.3%) |
| Genotype testing is important for patient care | 8 (61.5%) | 11 (84.6%) |
| EHR alerts are effective in supporting mood/anxiety management based on genotype | 8 (61.5%) | 10 (76.9%) |
| Genotype testing fits in well with how I already manage patients | 5 (38.5%) | 11 (84.6%) |
| My training has prepared me to use genotype information | 4 (30.8%) | 9 (69.2%) |
| Using genetic data to guide therapeutic choices improves my ability to prescribe medicine | 8 (61.5%) | 13 (100%) |
| Genotype testing improves ability to care for patients | 8 (61.5%) | 12 (92.3%) |
| Genotype testing is relevant to my clinical practice | 10 (76.9%) | 13 (100%) |
| I can find reliable sources of information about CYP2D6 and CYP2C19 genotype testing | 6 (46.2%) | 10 (76.9%) |
| CYP2D6 and CYP2C19 genotype testing should be available for clinical care | 10 (76.9%) | 11 (84.6%) |
| I have enough time to use genotype testing in clinical practice | 6 (46.2%) | 10 (76.9%) |
| I have trouble talking to my patients about CYP2D6 and CYP2C19 genotype testing | 3 (23.1%) | 0 (0%) |
EHR, electronic health record; PGx, pharmacogenetics.
Patients
Twenty‐five of 49 (51%) participating families completed the post‐survey questionnaire (Figure 1 ). In the pre‐survey (N = 49), parents expressed concerns related primarily to ethical and economic issues. Twenty percent (n = 10) of parents were concerned that PGx testing may hurt their child’s ability to get health or other insurance (e.g., life or disability insurance). Additionally, 16% (n = 8) of parents responding expressed concerns that PGx testing may affect their child’s employment opportunities in the future. Another concern from parents was that PGx testing might reveal a risk for certain disease (n = 10, 20%) and a few (n = 3, 6%) felt that the results could have the potential to adversely affect their family. However, none of the responders endorsed this concern in the post‐survey.
Most parents indicated in the pre‐survey that they were willing to cover expenses for PGx testing. The average cost parents were willing to pay was $235; 24% (n = 12) of parents endorsed paying $500 or more, whereas 14% (n = 7) stated that they would not be willing to pay anything for the test. In the post‐survey completed by 11 parents, 18% (n = 2) endorsed being willing to pay $500 or more for PGx testing, whereas most of the parents (72.7%, n = 8), endorsed paying $200 or less.
Most parents (94%, n = 46), agreed or strongly agreed that PGx testing could help their providers to choose better and safer medications for their child in the pre‐survey vs. 91% (n = 10) in the post‐survey, whereas 8% were neutral about this question (see Table S2 ). Fewer than half of respondents (47%, n = 23), at the beginning of the study were interested in PGx testing for future medications vs. 73% (n = 8) in the post‐survey.
Feasibility of PGx testing
Medication recommendations based on PGx testing results
Both the PGx testing results and the consult notes with recommendations from PMP pharmacists were available in the EHR. The mean time for obtaining PGx test results for the implementation arm was longer for CYP2D6 (11.4 days ± 10.4 days) than for CYP2C19 (8.4 days ± 8.8 days; t = 1.1, Pr(T> t) = 0.142). The mean time to having the consult notes with genotype‐guided recommendations in the EHR was 15 days (± 8.9 days). Physicians referred to PGx test results in their progress notes for 18 patients in the implementation group (72%). Only one physician did not acknowledge PGx test results for treatment decisions.
Utility of PGx testing
The distribution of CYP2D6 and CYP2C19 phenotypes are available in Table 3 . Three patients who were using bupropion (strong CYPD6 inhibitor) were phenoconverted to PMs. Twelve (48%) of patients in the implementation arm and 9 (37.5%) in the control arm had at least one actionable phenotype under the standard definition (i.e., PM or RM/UM; Table 4 ). Eleven of the patients with an actionable phenotype in the implementation arm had an SSRI prescription during the trial and 100% of these prescriptions or medication changes were concordant with their phenotype. Eight of the patients with an actionable phenotype in the control arm had an SSRI prescription and 75% were concordant. There was no difference in concordance rates between implementation and control arms (P = 0.16).
Table 3.
CYP2D6 and CYP2C19 phenotypes distribution across randomization groups
| Phenotype | CYP2D6, N (%) | CYP2C19, N (%) | ||
|---|---|---|---|---|
| Control (n = 24) | Implementation (n = 25) | Control (n = 24) | Implementation (n = 25) | |
| Poor metabolizer a | 4 (16.6) | 1 (4.0) | 1 (4.2) | 0 (0) |
| Intermediate metabolizer | 1 (4.2) | 2 (8.0) | 10 (41.7) | 6 (24.0) |
| Normal metabolizer | 18 (75.0) | 20 (80.0) | 9 (37.5) | 10 (40.0) |
| Rapid metabolizer | – | – | 4 (16.7) | 7 (28.0) |
| Ultra‐rapid metabolizer | 1 (4.2) | 2 (8.0) b | 0 (0) | 2 (8.0) |
Three individuals (one in the implementation and two in the control groups) were considered poor metabolizers as they were using a strong CYP2D6 inhibitor (bupropion).
One individual was a range phenotype (normal to ultra‐rapid metabolizer) but treated clinically as ultra‐rapid metabolizer.
Table 4.
Concordance rates across groups based on actionable phenotypes and use of SSRIs
| Controls (N = 24) | Implementation (N = 25) | |||
|---|---|---|---|---|
| CYP2D6, N (%) | CYPC19, N (%) | CYP2D6, N (%) | CYP2C19, N (%) | |
| Total N (% of total) with potentially actionable phenotypes | 5 a (20.8) | 5 a (20.8) | 3 (12.0) | 9 (36.0) |
| Total N (% of actionable) with concordant medication changes if prescribed a medication | 2 (50.0) d | 4 (80.0) | 3 (100.0) | 8 (100.0) d |
| Total N with discordant actionable phenotypes in relation to medications taken at baseline. Note: total N on medications at baseline = 38 | 1 | 3 | 0 | 4 |
| Medication change, N | ||||
| Medication metabolized by the other CYP enzyme; no change required | 2 | 1 | 3 | 4 |
| Medication changed to one metabolized by the other CYP enzyme | 0 | 1 b | 0 | 1 c |
| Dose of medication changed | 0 | 2 | 0 | 3 |
SSRIs, selective serotonin reuptake inhibitors.
One patient had both an actionable phenotype for CYP2D6 and for CYP2C19.
Rapid metabolizer originally on escitalopram, switched to fluoxetine.
Ultra‐rapid metabolizer originally on sertraline, switched to duloxetine.
One individual never prescribed an SSRI throughout the study and was not counted as being concordant nor discordant.
None of the clinical end points examined, either for the secondary outcomes (CIS and ASEC scores), or the post hoc analyses (CDI and SCARED scores), showed significant differences between the control and implementation arms at 12 weeks (Figure S1 ). Nonetheless, there were interesting observations across the whole cohort. Baseline mixed linear models (i.e., linear and quadratic time with group as the overall number of actionable phenotypes (0, 1, or 2) across both CYP2D6 and CYP2C19) revealed a statistically significant improvement in global impairment (CIS linear: −0.63, SE = 0.11, P < 0.01 and CIS quadratic: 0.07, SE = 0.02, P = 0.001) and antidepressant‐related side effects (ASEC linear: −0.51, SE = 0.11, P < 0.01 and ASEC quadratic 0.11, SE = 0.02, P < 0.001) from baseline to the eighth week, followed by a worsening for the next 4 weeks. Across the best fitting models, CYP2D6 PM/IM phenotypes experienced a steeper rate of improvement of ASEC scores (i.e., fewer side effects) from baseline to the eighth week, followed by a steeper rate of worsening for the next 4 weeks when compared with NMs and RM/UMs (ASEC linear*phenotype: 0.66, SE = 0.24, P = 0.01 and ASEC quadratic*phenotype: −0.17, SE = 0.04, P < 0.01; Figure 2 ). Similarly, CYP2C19 PM/IM phenotypes showed improvement in antidepressant‐related side effects as measured by the ASEC scores compared with NM, and to RM/UM phenotypes, who actually worsened with time (ASEC linear*phenotype: 0.32, SE = 0.14, P = 0.02; Figure 3 ). The models examining changes in impairment using CIS scores were further indicative of improvements in global impairments over time (CIS linear: −0.62, SE = 0.11, P < 0.01 and CIS quadratic: 0.07, SE = 0.02, P = 0.002), although, for this measure, the effects were not moderated by either CYP2D6 or CYP2C19 phenotypes.
Figure 2.
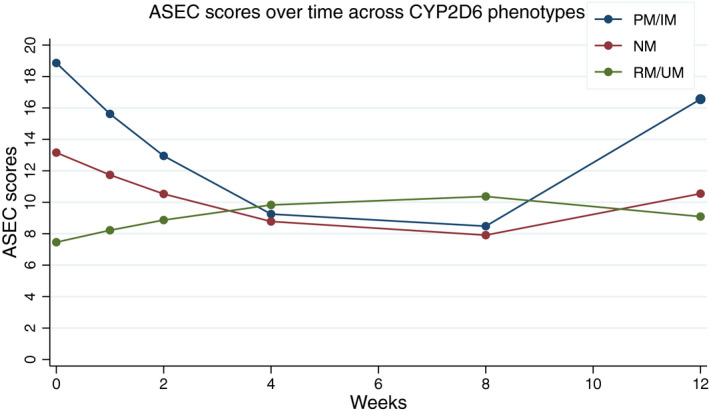
Antidepressant‐related adverse events as measured by mean Antidepressant Side‐Effect Checklist (ASEC) scores over time for CYP2D6. Higher scores indicate higher number of adverse events related to antidepressant medications. This predictive model shows that from week 0 to week 4, poor metabolizers (PMs) and intermediate metabolizers (IMs) showed a steeper rate of decrease in ASEC scores. From the fourth week to the eighth week, scores are relatively stable independent of CYP2D6 phenotype. PMs and IMs showed the highest change in ASEC scores (worsening) from week 8 to week 12, whereas there is only a slight increase in normal metabolizers (NMs). RM, rapid metabolizer; UM, ultra‐rapid metabolizer.
Figure 3.
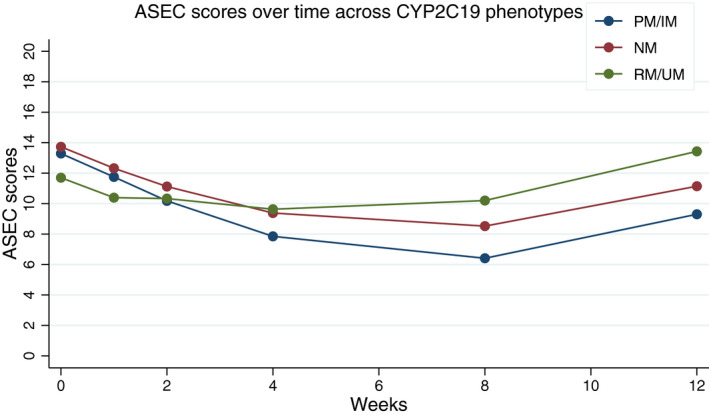
Antidepressant‐related adverse events as measured by mean Antidepressant Side‐Effect Checklist (ASEC) scores over time for CYP2C19. Higher scores indicate higher number of adverse events related to antidepressant medications. This predictive model shows that from week 0 to week 4, ASEC scores decrease independent of the CY2C19 phenotype and remain relatively stable from week 4 to week 8. Although the scores increase for all CYP2C19 phenotypes after the eighth week, the scores remain relatively higher for RMs and UMs. IM, intermediate metabolizer; NM, normal metabolizer; PM, poor metabolizer; RM, rapid metabolizer; UM, ultra‐rapid metabolizer.
DISCUSSION
Main findings
The findings of this study suggest that the implementation of PGx in a pediatric psychiatry clinic is both feasible and well accepted by families and physicians. Although parents were concerned at the beginning of the study about the societal and ethical implications of genetic testing, most agreed that PGx testing may help providers to choose more effective and safer medications for their children by the end of the study. Furthermore, most parents were willing to pay $100 to $200 for PGx testing. Unanimously, the physicians agreed in the post‐survey that having genetic data available improved their ability to select medications for their patients. The vast majority of physicians reported feeling confident in using genetic information.
There are several factors that may limit the feasibility of PGx testing, such as the lack of PGx education and/or knowledge of PGx testing availability, and how to apply genotype results to prescribing decisions. 24 Studies have consistently identified the need of physicians to receive education about PGx in order to interpret testing results and answer their patients’ questions. 25 , 26 For this study, participating physicians were enabled to use genetic information to guide their medical decisions through both formal education and ongoing clinical support provided by UF Health PMP pharmacists. Most of the participant physicians acknowledged the results from PGx testing and followed pharmacist‐provided recommendations based on genetic information.
Challenges and solutions
However, efficient provision of PGx results and consult notes with genotype‐guided recommendations was a major challenge in our study. The length of time required to obtain the results and place consult notes by pharmacists may have delayed or impeded the utilization of the results for medical decision making. Consequently, physicians may have started or changed a medication for their patients prior to genetic information being available. Genotype turnaround time has been identified as a feasibility concern of PGx testing in psychiatry because physicians may require the results in a short period of time for patients with severe clinical presentations. 27 We have subsequently reduced the total turnaround time; beginning in July 2019, our consult notes are placed between 1 to 3 days (average 3.85 days ± 6.91 days, median: 1.5 days) after the PGx test results are ready. Similarly, as a result of this study, which confirmed that children are often averse to invasive sample collection methods (e.g., blood draws), we have validated PGx testing using DNA from buccal swabs, 15 further facilitating the testing process.
Overall, we did not find statistically significant differences in any of the secondary clinical end points between the implementation and the control arms, although we observed some interesting differences by genotype. Perhaps most importantly, in our study, CYP2D6 PMs and IMs showed a rapid decrease in antidepressant‐related side effects up to week 8, whereas CYP2C19 PMs and IMs experienced a decrease throughout the weeks of the study compared with NMs and RMs and UMs. As the majority of participants were on medications prior to starting the study, the ASEC cannot effectively differentiate between baseline medication side effects and similar symptoms caused by the underlying mood or anxiety disorder. Future studies are needed to distinguish these effects from one another. However, in context of this study, the ASEC can be thought of as a global measure of symptom severity (regardless of etiology).
Consistent with our findings, Oshikoya and colleagues found that children with CYP2D6 PM and IM phenotypes had more adverse events when taking risperidone, 28 another CYP2D6 substrate. Similarly, Strawn and colleagues used a pharmacokinetics approach to demonstrate that CYP2C19 PM and IM pediatric patients had longer half‐lives and higher maximum plasma concentrations of escitalopram and sertraline. 29 Aldrich et al. found that the CYP2C19 PM and IM phenotypes were associated with a higher proportion of side effects and higher frequency of treatment (escitalopram and citalopram) discontinuation in youths. 11 Together, our findings underscore the potential opportunity for identifying patients through PGx testing who may experience antidepressant‐related adverse events with CYP2D6 and CYP2C19 substrate medications resulting in treatment discontinuation.
Limitations
There are several limitations for our study. First, our sample size was small given the pilot nature of the trial, and therefore was underpowered to detect significant differences in clinical outcomes between study arms; we were also underpowered to include concordance/discordance between PGx phenotype and baseline medications in the analyses. Second, only half of the participant families completed the post‐survey, and, therefore, the responses may not necessarily represent the attitudes of the whole cohort toward PGx testing. Nonetheless, the responses to the post‐survey overwhelmingly favored PGx testing. Third, as this study required a long follow‐up period (12 weeks), some of the intervening self‐reported clinical surveys to quantify symptoms severity and adverse events were not completed by the participant families and, consequently, some data were missing. Finally, the majority of participants were on medications at baseline, and we were not able to distinguish between changes in underlying disease symptomatology and medication side effects, which can often, especially in a pediatric population, overlap.
Significance and future projections
Our findings suggest that families and physicians have positive attitudes toward PGx testing to assist with selection of antidepressant medications for children and adolescents. However, the results of PGx testing should ideally be available early, before medication is started, to be considered by physicians in the treatment selection or dose adjustment and to maximize its potential for improving medication efficacy and reducing side effects. The findings from this small pragmatic trial were nevertheless important to informing the methodology of the National Human Genome Research Institute (NHGRI)‐funded Implementing GeNomics In pracTicE (IGNITE) Pragmatic Clinical Trials Network, 30 a multi‐site trial funded by the NHGRI. There are three pharmacogenetic trials being conducted by IGNITE, including one that aims to investigate the use of PGx testing to guide SSRI prescribing in adult and pediatric populations.
Funding
This study was funded by the National Institutes of Health IGNITE Network (NIH grant U01 HG007269 to Dr. Julie Johnson) and the UF Clinical and Translational Science Institute (CTSI), which is supported by the NIH National Center for Advancing Translational Sciences (NCATS) (UL1TR001427). Dr. Karla Claudio‐Campos is supported by T32HG008958 by the National Genome Research Institute (NHGRI).
Conflict of Interest
The authors declared no competing interests for this work.
Author Contributions
K.C., A.P., G.J., and E.J.C. wrote the manuscript. C.A.M., L.H.C., and K.W. designed the research. J.N., R.N., A.M., D.M.S., Y.S., and M.M. performed the research. A.P. and K.C. analyzed the data.
Supporting information
FigS1
TableS1
TableS2
Acknowledgments
The authors thank the participants and families who contributed their information and time so that this study could be conducted. We would like to acknowledge the trainee Dr. Benjamin Duong who contributed to this study by writing consult notes. We also would like to acknowledge Dr. Julie Johnson for her support to our research group.
References
- 1. Cree, R.A. et al. Health care, family, and community factors associated with mental, behavioral, and developmental disorders and poverty among children aged 2–8 years — United States 2016. Morb. Mortal. Wkly. Rep. 50, 1377–1383 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ghandour, R.M. et al. Prevalence and treatment of depression, anxiety, and conduct problems in US children. J. Pediatr. 206, 256–267.e253 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bridge, J.A. et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta‐analysis of randomized controlled trials. JAMA 297, 1683–1696 (2007). [DOI] [PubMed] [Google Scholar]
- 4. Locher, C. et al. Efficacy and safety of selective serotonin reuptake inhibitors, serotonin‐norepinephrine reuptake inhibitors, and placebo for common psychiatric disorders among children and adolescents: a systematic review and meta‐analysis. JAMA Psychiatr. 74, 1011–1020 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Strawn, J.R. , Mills, J.A. , Sauley, B.A. & Welge, J.A. The impact of antidepressant dose and class on treatment response in pediatric anxiety disorders: a meta‐analysis. J. Am. Acad. Child Adolescent Psychiatr. 57, 235–244.e232 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shehab, N. et al. US emergency department visits for outpatient adverse drug events, 2013–2014. JAMA 316, 2115–2125 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stevens, J. et al. Parental attitudes toward children's use of antidepressants and psychotherapy. J. Child Adolesc. Psychopharmacol. 19, 289–296 (2009). [DOI] [PubMed] [Google Scholar]
- 8. Roden, D.M. et al. Pharmacogenomics. Lancet 394, 521–532 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stingl, J.C. , Brockmöller, J. & Viviani, R. Genetic variability of drug‐metabolizing enzymes: the dual impact on psychiatric therapy and regulation of brain function. Mol. Psychiatr. 18, 273–287 (2013). [DOI] [PubMed] [Google Scholar]
- 10. Maruf, A.A. , Greenslade, A. , Arnold, P.D. & Bousman, C. Antidepressant pharmacogenetics in children and young adults: a systematic review. J. Affect Disord. 254, 98–108 (2019). [DOI] [PubMed] [Google Scholar]
- 11. Aldrich, S.L. et al. Influence of CYP2C19 metabolizer status on escitalopram/citalopram tolerability and response in youth with anxiety and depressive disorders. Front. Pharmacol. 10, 99 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gassó, P. et al. Effect of CYP2D6, CYP2C9 and ABCB1 genotypes on fluoxetine plasma concentrations and clinical improvement in children and adolescent patients. Pharmacogenom. J. 14, 457–462 (2014). [DOI] [PubMed] [Google Scholar]
- 13. AlOlaby, R.R. et al. Molecular biomarkers predictive of sertraline treatment response in young children with fragile X syndrome. Brain Develop. 39, 483–492 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thieme, D. , Rolf, B. , Sachs, H. & Schmid, D. Correlation of inter‐individual variations of amitriptyline metabolism examined in hairs with CYP2C19 and CYP2D6 polymorphisms. Int. J. Legal Med. 122, 149–155 (2008). [DOI] [PubMed] [Google Scholar]
- 15. Cicali, E.J. et al. Challenges and lessons learned from clinical pharmacogenetic implementation of multiple gene‐drug pairs across ambulatory care settings. Genet. Med. 21, 2264–2274 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hicks, J.K. et al. Clinical pharmacogenetics implementation consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin. Pharmacol. Therapeut. 98, 127–134 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caudle, K.E. et al. Standardizing CYP2D6 genotype to phenotype translation: consensus recommendations from the clinical pharmacogenetics implementation consortium and Dutch pharmacogenetics working group. Clin. Transl. Sci. 13, 116–124 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saiz‐Rodríguez, M. et al. Effect of polymorphisms on the pharmacokinetics, pharmacodynamics and safety of sertraline in healthy volunteers. Basic Clin. Pharmacol. Toxicol. 122, 501–511 (2018). [DOI] [PubMed] [Google Scholar]
- 19. Bird, H.R. et al. The Columbia Impairment Scale (CIS): pilot findings on a measure of global impairment for children and adolescents. Int. J. Methods Psychiatric Res. 3, 167–176 (1993). [Google Scholar]
- 20. Uher, R. et al. Adverse reactions to antidepressants. Br. J. Psychiatr. 195, 202–210 (2009). [DOI] [PubMed] [Google Scholar]
- 21. Kovacs, M. Children's Depression Inventory. A measure of depressive symptoms in young persons. (1992).
- 22. Foa, E.B. et al. The obsessive‐compulsive inventory: development and validation of a short version. Psychol. Assess. 14, 485–496 (2002). [PubMed] [Google Scholar]
- 23. Birmaher, B. et al. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. J. Am. Acad. Child. Adolesc. Psychiatr. 38, 1230–1236 (1999). [DOI] [PubMed] [Google Scholar]
- 24. Deininger, K.M. et al. National survey of physicians’ perspectives on pharmacogenetic testing in solid organ transplantation. Clin. Transplant. https://doi.org/ 10.1111/ctr.14037. [DOI] [PubMed] [Google Scholar]
- 25. Christensen, K.D. et al. Are physicians prepared for whole genome sequencing? A qualitative analysis. Clin. Genet. 89, 228–234 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Powell, K.P. et al. Educational needs of primary care physicians regarding direct‐to‐consumer genetic testing. J. Genet. Couns. 21, 469–478 (2012). [DOI] [PubMed] [Google Scholar]
- 27. Maruf, A.A. et al. Pharmacogenetic testing options relevant to psychiatry in Canada. Can. J. Psychiatr. 65, 521–530 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oshikoya, K.A. et al. CYP2D6 genotype and adverse events to risperidone in children and adolescents. Pediatr. Res. 85, 602–606 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Strawn, J.R. , Poweleit, E.A. & Ramsey, L.B. CYP2C19‐guided escitalopram and sertraline dosing in pediatric patients: a pharmacokinetic modeling study. J. Child Adolesc. Psychopharmacol. 29, 340–347 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. National Institute of Health, National Human Genome Research Institute . Implementing Genomics in Practice (IGNITE) II: Pragmatic Clinical Trials Network (2020). June 24, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FigS1
TableS1
TableS2


