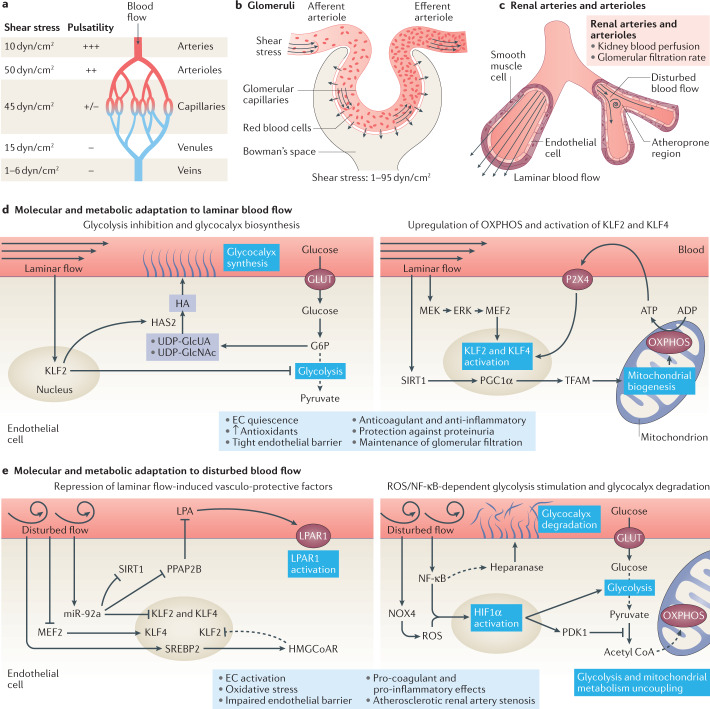
Fig. 5. Response of the renal endothelium to changes in blood flow and shear stress.
a | Arteries, arterioles, capillaries, venules and veins are exposed to different types of blood flow as defined by its pulsatility and the level of shear stress. b | Glomerular capillaries are exposed to high shear stress as a result of high blood flow and pressure together with increasing blood viscosity due to the filtration process. c | Some regions of renal arteries and arterioles — which control kidney blood perfusion and glomerular filtration rate — are exposed to laminar blood flow, whereas other regions — particularly regions of vessel bifurcation — are exposed to disturbed blood flow. Regions of disturbed blood flow are more likely to develop atherosclerotic lesions. d | Endothelial cells (ECs) exposed to laminar blood flow inhibit glycolytic metabolism in a KLF2-dependent manner and divert glycolytic intermediates to pathways involved in glycocalyx biosynthesis (left panel). Laminar flow also induces SIRT1–PGC1α–TFAM-mediated mitochondrial biogenesis, and stimulates the production of oxidative phosphorylation (OXPHOS)-derived ATP, which activates purinergic P2X4 receptors and further induces the activation of KLF2 and KLF4 transcription factors. Moreover, Krüppel-like factor 2 (KLF2) and KLF4 are upregulated in response to MEK5–ERK5 pathway activation (right panel). Laminar blood flow promotes EC quiescence, the production of antioxidants, the formation of a tight endothelial barrier and the acquisition of an anticoagulant and anti-inflammatory phenotype, and protects against proteinuria while maintaining glomerular filtration. e | Disturbed blood flow represses the protective pathways induced by laminar blood flow. Induction of microRNA (miR)-92a by disturbed flow represses the endothelial expression of KLF2, KLF4 and SIRT1 and PPAP2B, releasing the inhibitory effect of PPAP2B on circulating lysophosphatidic acid (LPA). Binding of LPA to its receptor LPAR1 induces pro-inflammatory signalling. The induction of SREBP2 upregulates HMG-CoA reductase, which increases intracellular cholesterol levels, and further upregulates miR-92a. Hypermethylation of the KLF4 promoter prevents MEF2 binding and subsequent KLF4 transcription (left panel). ECs exposed to disturbed blood flow also demonstrate an uncoupling of glycolysis from mitochondrial metabolism, with an upregulation of glycolysis. Disturbed flow also induces the production of NOX4-derived reactive oxygen species (ROS) and activates nuclear factor κB (NF-κB), leading to the upregulation and stabilization of hypoxia inducible factor 1α (HIF1α). Activation of the NF-κB pathway has been linked to heparanase activity and consequent glycocalyx degradation (right panel). Disturbed blood flow ultimately triggers EC activation and oxidative stress, impairs the endothelial barrier, induces a procoagulant and pro-inflammatory EC phenotype, and favours the development of atherosclerotic renal artery stenosis. ERK, extracellular signal regulated kinase; G6P, glucose-6-phosphate; GLUT, glucose transporter; HA, hyaluronan; HAS2, hyaluronan synthase 2; HMGCoAR, hydroxymethylglutaryl CoA reductase; MEF2, myocyte enhancer factor-2; MEK, mitogen-activated protein kinase kinase; NOX4, NADPH oxidase 4; PDK1, pyruvate dehydrogenase kinase 1; PGC1α, peroxisome proliferator-activated receptor gamma coactivator-1α; PPAP2B, phosphatidic acid phosphatase type 2B; SIRT1, sirtuin 1; SREBP2, sterol regulatory element binding protein 2; TFAM, mitochondrial transcription factor A; UDP-GlcNAc, uridine diphosphate-N-acetylglucosamine; UDP-GlcUA, uridine diphosphate–glucuronic acid.