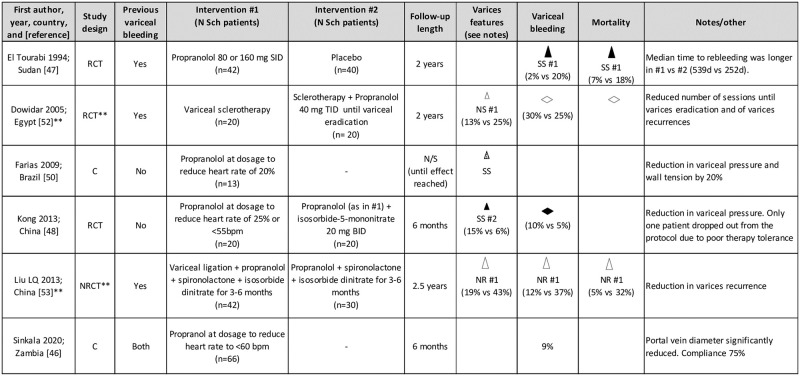
Fig 3. Direction effect chart summary of the included studies investigating the use of beta-blockers for the clinical management of hepatosplenic schistosomiasis.
Evaluation of all outcomes refers to the end of follow-up. **Data extracted from abstract. Sch, schistosomiasis. C, Cohort study. C–C, Case–Control study. RCT, Randomized Clinical Trial. NRCT, Non-Randomized Clinical Trial. N/S, not specified. Triangle orientation indicates direction of outcome: upward = amelioration in respect to other intervention or baseline, horizontal = no difference between interventions or from baseline. Triangle size indicates sample size per (smallest) group: small ≤20 pts, medium 21–49 pts, large ≥50 pts. Triangle color indicates quality of result based on study design and source: black = RCT, light gray = C–C, dotted = C, white = data from abstract. SS, statistically significant, NS, not statistically significant, NR, statistical analysis not reported. #n = intervention indicated in the corresponding “Intervention #n” column of the table to which the outcome direction refers [46–48,50,52,53].