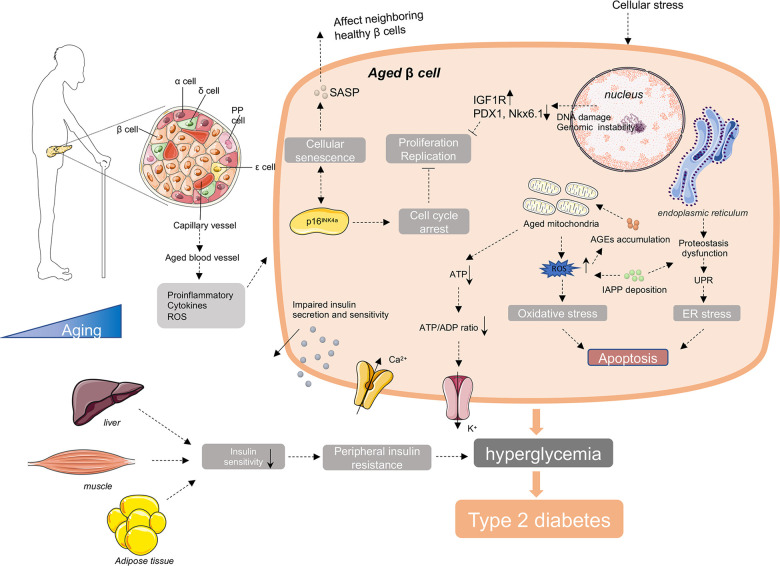
Figure 2.
Molecular mechanisms involving in age-related diabetes. In the process of aging, there are increased accumulation of ROS, unfolded protein, DNA damage, IAPP, AGEs and other cellular stress in aged β cells. These intracellular stresses make β cells more susceptible to apoptosis. And with age, there are increased cell cycle inhibitors, such as p16INK4a, and decreased cell cycle activators, such as CDK4 and CDK6 in β cells. These changes lead to the reduce of the proliferation and regeneration potential and induce the cellular senescence of β cells. Senescent β cells secrete a series of senescence-associated secretory phenotype (SASP), promoting the senescence of neighboring healthy β cells through induction of paracrine senescence. In addition, islet blood vessels are undergoing aging as well. Oxidative damage, inflammation and fibrosis in islet blood vessels may disturb β cell function. Besides islets, the external factors include the reduction of insulin sensitivity in peripheral insulin responsive tissues with advanced age, responsible for the increased demand for insulin and final exhaustion of β cells. Taken together, the decreased insulin secretion capacity of β cells and increased insulin resistance with age lead to the failure of glucose control in elderly body, and ultimately the onset and development of age-related diabetes.