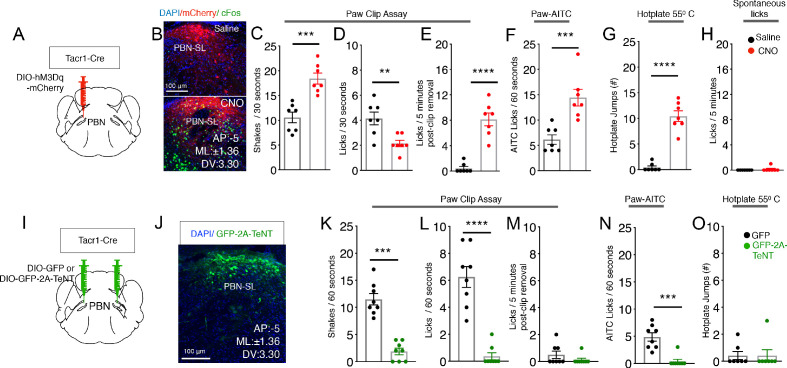
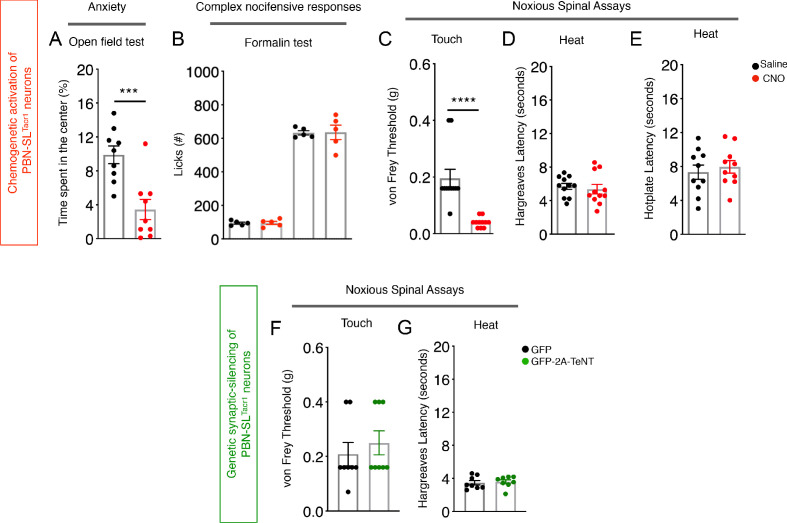
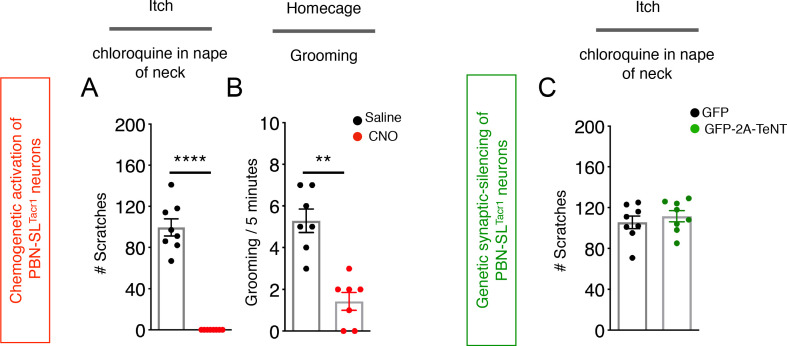
Figure 5. PBN-SLTacr1 neurons are necessary and sufficient to drive pain-related behaviors.
(A–H) Chemogenetic activation of PBN-SLTacr1 neurons results in hyperalgesia. (A) A cartoon depicting the strategy for stimulation of PBN-SLTacr1 neurons. The PBN of a Tacr1Cre mouse was injected with a Cre-dependent viral vector encoding an excitatory DREADD receptor (AAV-DIO-hM3Dq-mCherry) to allow chemogenetic activation. (B) Typical confocal images showing coronal sections of the PBN region from transduced Tacr1Cre mice treated with saline (top image) or CNO (bottom image). The expression of mCherry in both animals is restricted to select neurons in the PBN-SL (red). CNO application but not saline injection results in induction of the activity-dependent gene Fos (green) validating the approach. Scale = 100 μm. (C–G) Chemogenetic stimulation of PBN-SLTacr1 neurons heightens behavioral responses sustained by noxious mechanical (C–E), chemical (F), and thermal (G) stimulation (n = 7 mice tested with saline, black points and then with CNO, red points on different days). Mice treated with CNO spend significantly more time shaking (C; p=0.0003) and as a result exhibited less licking events (D; p=0.0044) directed to the hindpaw during the Clip assay. Notably, CNO-treated mice continued to lick the hindpaw for several minutes after the clip was removed (E; p=<0.0001). CNO treatment also increased the number of times mice licked their paws after topical AITC treatment (F; p=0.0009) and jumped to escape from a 55°C hotplate (G; p=<0.0001). CNO treatment did not increase result in licking behaviors in the absence of noxious stimulation (H; 0.3370). (I–O) Silencing of PBN-SLTacr1 neurons inhibits normal pain responses to sustained noxious stimuli. (I) A cartoon depicting the strategy for silence the output of PBN-SLTacr1 neurons. Bilateral injection of a Cre-dependent viral vector encoding Tetanus Toxin Light C GFP fusion (AAV-DIO-GFP-2A-TeNT) in Tacr1Cre mice was used to silence PBN-SLTacr1 neurons; AAV-DIO-GFP served as a control. (J) Typical confocal image of a coronal section through the PBN region showing the restricted expression of GFP-2A-TeNT in the PBN-SL (green). Scale = 100 μm. (K–O) Silencing of PBN-SLTacr1 neuronal output significantly dampened behavioral responses to sustained noxious mechanical (K–M), chemical (N) and thermal (O) stimulation (n = 8 GFP, black points; n = 7 TeNT-GFP, green points). In the Clip (sustained pinch assay, K–M), expression of GFP-2A-TeNT in PBN-SLTacr1 neurons decreases the time spent shaking (K; p=0.0003) and the number of licking events (L; p=<0.0001). Similarly, silencing PBN-SLTacr1 neurons significantly decreased the number of times mice licked their paws after topical AITC treatment (N; p=0.0001); since control animals did not make escape attempts from a 55°C hot plate (O; p=>0.9999) no effect of neuronal silencing was observed in this assay. Unpaired two-tailed t test; ns > 0.05, ***p≤0.001, ****p≤0.0001.