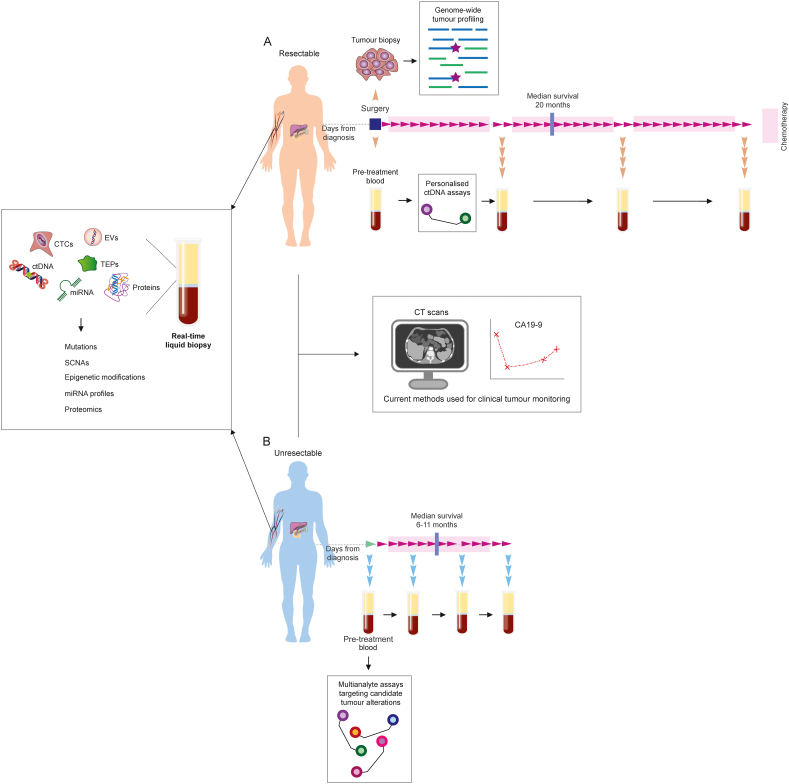
Fig. 5.
Longitudinal monitoring of PDAC tumours using multianalyte liquid biopsies. (A) Longitudinal monitoring of tumour response to adjuvant treatment, or disease progression following surgical primary tumour resection, is currently performed using routine imaging and by tracking levels of the tumour marker CA19-9 in blood. These measurements can be complemented using liquid biopsy approaches. In resectable cases with matched tumour biopsies available for genomic profiling, personalised assays can be developed to enhance the sensitivity for detection and tracking of rare mutant molecules in blood. (B) New and effective means of monitoring treatment response in patients with unresectable PDAC remains of significant clinical need. In the absence of resected tissue samples for tumour genotyping, multi-analyte liquid biopsy approaches, combining ctDNA and/or CTC detection with the analysis of miRNA and proteins in blood, present an alternative method to enhance the sensitivity for detection of tumour molecules. Unresectable patients also typically have a greater tumour burden and increased levels of circulating tumour molecules in peripheral blood, compared to patients with earlier stages of resectable disease, making them suitable candidates for combined orthogonal sampling and multi-marker profiling. CTC, circulating tumour cell; miRNA, microRNA, SCNA, somatic copy number alterations; TEP, tumour educated platelets.