Figure 1. Mitochondrial disease therapeutic pipeline development.
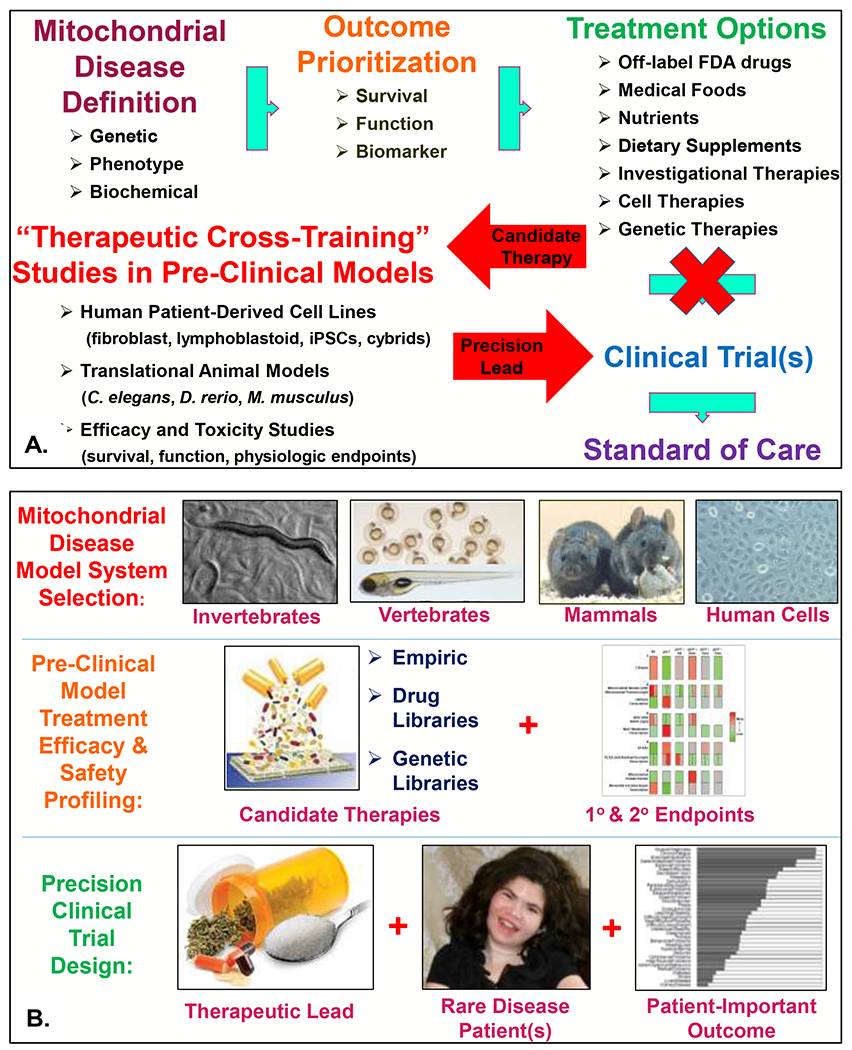
(A) The role of pre-clinical, translational research investigations in mitochondrial disease animal and cellular models to guide improved clinical trial design (modified from original publication) (Falk 2017). IPSCs, induced pluripotent stem cells. Cybrids, transmitochondrial hybrid lines that are the gold-standard for establishing the functional pathogenicity of mitochondrial DNA heteroplasmic variants (Giles et al 1980). (B) Schematic of ‘therapeutic cross-training” approach to support development of precision mitochondrial medicine therapies, utilizing C. elegans (worm), D. rerio (zebrafish), M. musculus (mouse), and H. sapiens (patient cells) to provide rigorous pre-clinical data in different species to optimize lead therapies and outcomes that support improved clinical trial design. The schematic in the middle right panel showing summarized interpretation of primary and secondary outcome measures assayed in a complex I C. elegans model summarizes data was originally published by our research group (McCormack et al 2015), as was the original data summarized in the bottom right panel indicating mitochondrial disease patient-prioritized outcomes (Zolkipli-Cunningham et al 2018). The patient displayed in the bottom center panel is a 16-year-old girl with FBXL4-based primary mitochondrial disease (Gai et al 2013), with photo shared per family consent per CHOP Institutional Review Board-approved study #08-6177 (Falk, PI).
