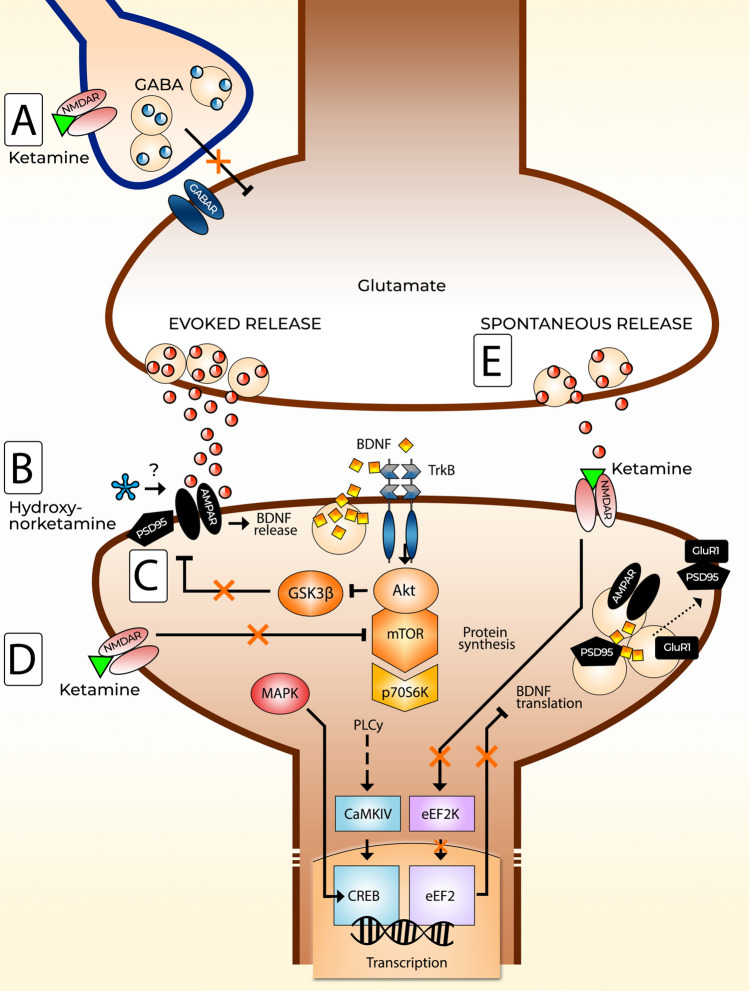
Fig. 3.
An overview of some of the proposed molecular mechanisms underlying ketamine’s rapid antidepressant action. a The disinhibition hypothesis. Ketamine preferentially blocks N-methyl-d-aspartate receptors (NMDARs) on gamma-aminobutyric acid (GABA)-ergic inhibitory interneurons, leading to a decrease in the inhibitory tone exerted on excitatory pyramidal neurons. Increased glutamate release acts on postsynaptic α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptors (AMPARs) and induces cellular effects such as the release of brain-derived neurotrophic factor (BDNF) and activation of its receptor tropomyosin receptor kinase B (TrkB) and the regulation of downstream pathways important for synaptic plasticity and protein synthesis. Downstream effects include activation of mitogen-activated protein kinase (MAPK) and mammalian target of rapamycin (mTOR) and regulation of AMPAR dynamics and scaffolding proteins such as postsynaptic density protein 95 (PSD95). b Hydroxynorketamine metabolites may modulate postsynaptic AMPAR signaling, leading to various downstream effects. c Inhibition of glycogen synthase kinase 3 β (GSK3β) by ketamine reduces phosphorylation of PSD95, which augments AMPAR signaling by reducing the internalization of AMPAR subunits, among other effects. d Ketamine may also block extrasynaptic NMDARs, normally tonically activated by glutamate, and induce mTOR activity. e The blockade of spontaneous NMDAR-mediated neurotransmission can have effects that lead to the disinhibition of BDNF translation via eukaryotic elongation factor 2 (eEF2)-dependent mechanisms