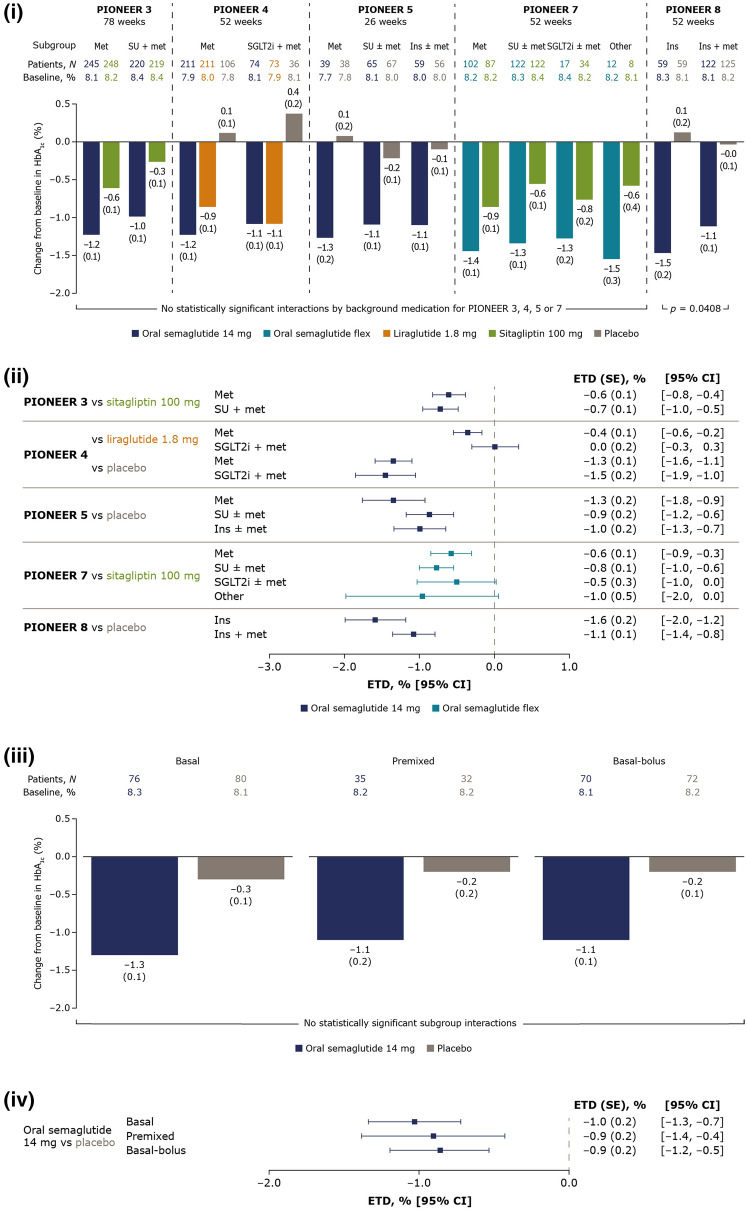
Fig. 1.
Change from baseline in HbA1c and estimated treatment differences for oral semaglutide 14 mg and flexibly dosed versus comparators by background medication (i and ii) and by insulin regimen (iii and iv). (i) Data are estimated changes from baseline (means with SEs in brackets) for the trial product estimand (on trial product without rescue medication). The p value is for the unadjusted two-sided test of the treatment by subgroup interaction. N number of patients contributing to the analysis, Other includes thiazolidinediones and other oral glucose-lowering medications. (ii) Data are for the trial product estimand (assumes patients remained on the trial product without rescue medication use). (iii) Data are estimated changes from baseline (means with SEs in brackets) for the treatment policy estimand (regardless of trial product discontinuation or rescue medication use in all randomised patients). N total number of patients in each subgroup (full analysis set). (iv) Data are for the treatment policy estimand. CI confidence interval, ETD estimated treatment difference, flex flexible dose adjustment, HbA1c glycated haemoglobin, ins insulin, met metformin, SE standard error of the mean, SGLT2i sodium-glucose cotransporter-2 inhibitor, SU sulphonylurea