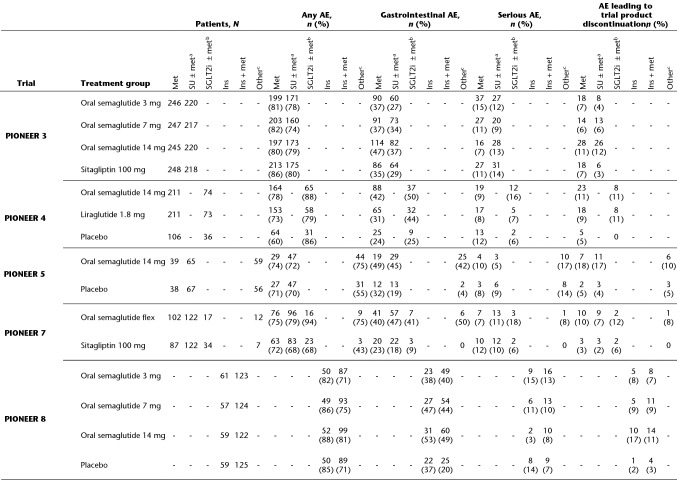
Table 3.
On-treatment adverse events
Gastrointestinal AEs were defined by MedDRA version 20.1 and comprised events of nausea, diarrhoea, vomiting, abdominal pain, constipation, gastroesophageal reflux disease, abdominal discomfort, dyspepsia, gastritis, abdominal pain upper, abdominal distention, dry mouth, flatulence, large intestine polyp, chronic gastritis, umbilical hernia, Barrett’s oesophagus, and dental caries
AE adverse event, flex flexible dose adjustment, ins insulin, met metformin, N number of patients included in analyses, n number of patients with at least one event, SGLT2i sodium-glucose cotransporter-2 inhibitor, SU sulphonylurea, TZD thiazolidinedione
aSU + met in PIONEER 3, SU ± met in PIONEER 5 and 7
bSGLT2i + met in PIONEER 4, SGLT2i ± met in PIONEER 7
cMet, SU ± met, or ins ± met in PIONEER 5, 1–2 of met, SU, SGLT2i or TZD in PIONEER 7