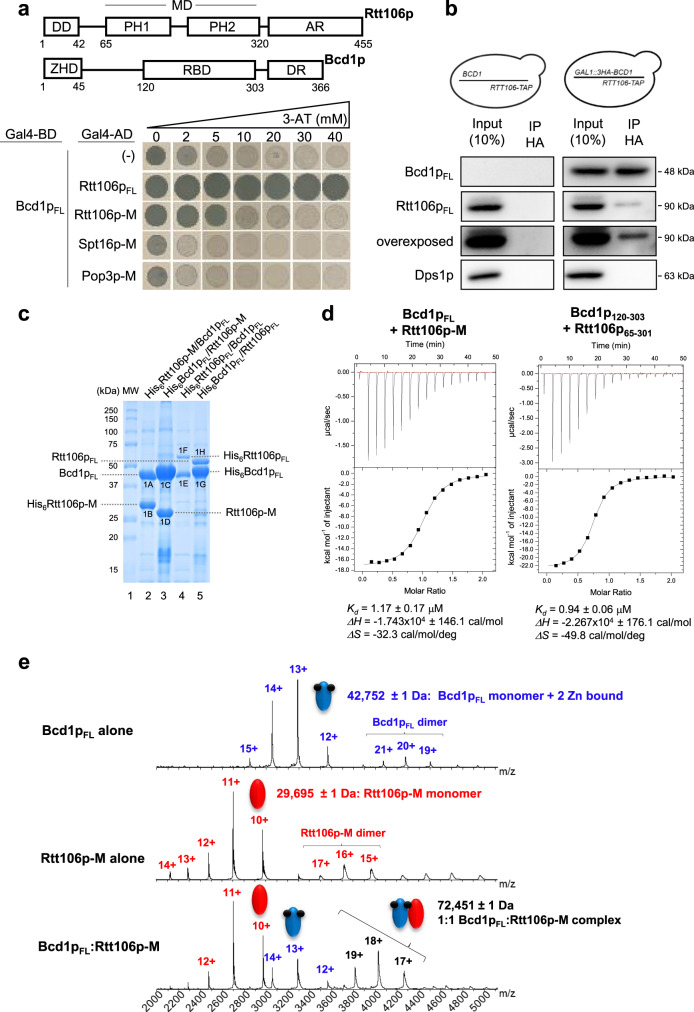
Fig. 2. Bcd1p and histone chaperone Rtt106p form a stable heterodimer.
a Schematic representation of the Rtt106p and Bcd1p domain organization. DD dimeric domain, PH Plekstrin homology, PH1 + PH2 = MD middle domain, AR acidic region, ZHD zinc finger HIT domain, RBD Rtt106 binding domain, DR disorder region. Y2H assay: full-length Bcd1p (Bcd1pFL) fused to the Gal4 binding domain (BD) interacts with the full-length Rtt106p (Rtt106pFL) or fragment spanning amino acids 65–320 (Rtt106p-M) fused to the Gal4 activation domain (AD) as evidenced by growth on a His deprived medium supplemented with increased concentrations of 3-amino-1, 2, 4-triazol (3-AT). No interaction was observed with homologous fragments of FACT chaperone components Spt16p and Pob3p (Spt16p-M and Pob3p-M). b Interaction of Bcd1pFL with Rtt106pFL in yeast. Co-immunoprecipitation (co-IP) was performed on GAL1::3HA-BCD1 × RTT106-TAP cells expressing 3xHA-tagged Bcd1pFL and TAP-tagged Rtt106pFL. Cells expressing the nontagged Bcd1p were used as negative control. Extracts were incubated with anti-HA beads. The co-immunoselected proteins were analyzed by SDS-PAGE and western blotting. 10% of total proteins used per assay were loaded in the input lane. Tagged proteins were detected with PAP antibodies for Rtt106p and anti-HA antibodies for Bcd1p. The Dps1 protein used to control protein loading was detected using specific anti-Dps1p antibodies. The two panels correspond to a cropping of two sections of the same membrane. The full-length membrane is presented in the Source data file. The experiment was independently repeated three times with similar results. c Interaction of recombinant Bcd1p and Rtt106p in E. coli. His6-tagged full-length or M domain of Rtt106p were co-expressed with Bcd1pFL. His6-tagged Bcd1pFL was co-expressed with Rtt106pFL or Rtt106p-M. Complexes were selected from crude extract by immobilized metal ion affinity chromatography (IMAC), fractionated by SDS-PAGE and revealed by Coomassie blue staining. The results correspond to co-expression with high salt (400 mM) buffer. Molecular weight markers (MW) in kilo Dalton (kDa) were loaded on the left. The experiment was repeated twice with similar results. The identity of the proteins in bands 1 A, 1B, 1 C, 1D, 1E, 1 F, 1 G, and 1H was confirmed by in-gel digestion of gel slices and mass spectrometry (MS) analysis of the peptide extract (Supplementary Table 1). d Bcd1p and Rtt106p interacting domains. ITC data for the interaction of Bcd1pFL with Rtt106p-M (on the left) and Bcd1p120-303 with Rtt106p65-301 (on the right) recorded at 293 K in buffer containing 10 mM NaPi at pH 7.5, 150 mM NaCl and 0.5 mM TCEP. The calculated affinities Kd, and thermodynamic parameters as variations in enthalpy (ΔH) and entropy (ΔS) are indicated. e Nondenaturing MS characterization of the complex formed upon incubation of recombinant Bcd1pFL with fragment Rtt106p-M. NanoESI mass spectra performed under nondenaturing conditions confirmed the presence of a 1:1 binding stoichiometry of Bcd1pFL:Rtt106p-M complex (Da = Dalton). Source data for panel b are provided as a Source Data file.