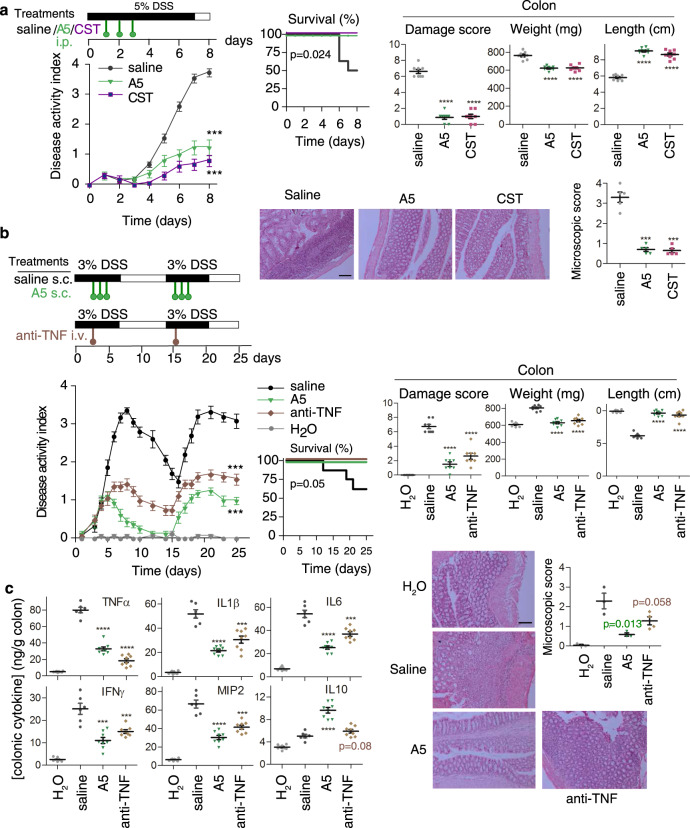
Fig. 3. Therapeutic effect of analogue 5 on DSS-induced ulcerative colitis.
a Protective effects against acute severe colitis induced by oral DSS by the treatment with Cortistatin (CST) and analogue 5 (A5) (as indicated in the scheme) evaluated by disease activity indexes (scoring body weight loss, stool consistency and presence of faecal blood), survival rate, macroscopic signs of colon inflammation (damage score, length and weight of colon) and histopathological scores. b Comparative therapeutic effects of treatments with A5 and anti-mouse TNFα antibody in a model of relapsing-remitting colitis induced by cyclic administration of DSS (see scheme for experimental design). c Effects of A5 and anti-TNFα treatments on levels of cytokines in colonic mucosa of mice with DSS-induced relapsing-remitting colitis. Mice receiving tap water instead of DSS were used as naive controls. Animals injected with saline instead of A5 were used as untreated colitic mice. n = 8 mice/group for all assays, unless for histological analysis (n = 5 mice/group in panel a, and n = 3 mice/group in panel b, where representative images are shown at ×100 magnification, scale bar: 200 µm). Data are mean ± SEM with dots representing individual values of biologically independent animals. Statistical differences between groups were calculated using two-tailed non-parametric Mann–Whitney test (for disease activity index, colon damage and microscopic scores), unpaired two-tailed Student’s t test (for colon length and weight) and Kaplan–Meier test (for survival). ***p < 0.001; ****p < 0.0001 versus untreated DSS-colitic mice (saline). Exact p-values are shown for p > 0.001. Source data are provided as a Source Data file. i.p. intraperitoneal, s.c. subcutaneous, i.v. intravenous.