Abstract
Background
Recent randomised trials showed benefit for anti-inflammatory therapies in coronary disease but excluded stroke. The prognostic value of blood inflammatory markers after stroke is uncertain and guidelines do not recommend their routine measurement for risk stratification.
Methods
We performed a systematic review and meta-analysis of studies investigating the association of C-reactive protein (CRP), interleukin-6 (IL-6) and fibrinogen and risk of recurrent stroke or major vascular events (MVEs). We searched EMBASE and Ovid Medline until 10/1/19. Random-effects meta-analysis was performed for studies reporting comparable effect measures.
Results
Of 2,515 reports identified, 39 met eligibility criteria (IL-6, n = 10; CRP, n = 33; fibrinogen, n = 16). An association with recurrent stroke was reported in 12/26 studies (CRP), 2/11 (fibrinogen) and 3/6 (IL-6). On random-effects meta-analysis of comparable studies, CRP was associated with an increased risk of recurrent stroke [pooled hazard ratio (HR) per 1 standard-deviation (SD) increase in loge-CRP (1.14, 95% CI 1.06–1.22, p < 0.01)] and MVEs (pooled HR 1.21, CI 1.10–1.34, p < 0.01). Fibrinogen was also associated with recurrent stroke (HR 1.26, CI 1.07–1.47, p < 0.01) and MVEs (HR 1.31, 95% CI 1.15–1.49, p < 0.01). Trends were identified for IL-6 for recurrent stroke (HR per 1-SD increase 1.17, CI 0.97–1.41, p = 0.10) and MVEs (HR 1.22, CI 0.96–1.55, p = 0.10).
Conclusion
Despite evidence suggesting an association between inflammatory markers and post-stroke vascular recurrence, substantial methodological heterogeneity was apparent between studies. Individual-patient pooled analysis and standardisation of methods are needed to determine the prognostic role of blood inflammatory markers and to improve patient selection for randomised trials of inflammatory therapies.
Keywords: Ischaemic stroke, transient ischaemic attack, recurrence, interleukin-6, C-reactive protein, fibrinogen, prognosis, inflammation
Introduction
Despite the widespread use of modern secondary prevention therapy, there is a significant residual vascular risk in ischaemic stroke survivors, with rates of recurrent stroke and MVEs of 25–30% at 5 years.1 There is an urgent need for new therapeutic targets to reduce this residual risk after stroke.
Inflammation has a central role in atherosclerosis pathogenesis, stroke, and coronary events.2–4 Plaque inflammation imaged by positron emission tomography (PET) predicts early recurrent stroke in patients with symptomatic carotid stenosis.5 CRP and fibrinogen are associated with increased risk of first stroke and coronary events in apparently-healthy individuals.6,7 Mendelian randomisation and genetic epidemiological studies indicate increased risk of first stroke associated with higher expression of monocyte chemoattractant protein-1 (MCP-1) and enhanced IL-6 receptor signalling.8,9 In randomised trials (RCTs) of patients with coronary disease, the risk of major vascular events was reduced in patients treated with canakinumab (an interleukin-1β inhibitor) and colchicine (a tubulin inhibitor with pleotropic anti-inflammatory effects).10–12
Despite the growing evidence linking inflammation to vascular risk, the prognostic role of inflammatory markers after stroke remains unclear. Studies which have investigated this question have reported contradictory findings and current guidelines do not recommend routine measurement of inflammatory markers for risk prediction after stroke. Improved data are needed to guide clinical practice and improve selection of patients for clinical trials of anti-inflammatory therapy. We performed a systematic review of studies investigating the association between IL-6, CRP, and fibrinogen, measured after ischaemic stroke or transient ischaemic attack (TIA), and the risk of recurrent stroke and major vascular events.
Patients and methods
Systematic review protocol and manuscript preparation
The systematic review protocol was published in advance on PROSPERO (registration number: CRD42018116190) and the manuscript was written according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.13
Search strategy and study eligibility
Eligible studies: 1) Had 50 patients or more with ischaemic stroke or TIA; 2) Blood samples analysed for CRP and/or IL-6 and/or fibrinogen, measured after the index event; 3) Reported associations with recurrent stroke (ischaemic or haemorrhagic stroke) or MVEs (defined as any combination of myocardial infarction, unstable angina, coronary revascularisation, or vascular death); 4) Included cohort studies, case-control studies, or observational data taken from RCTs. Key study exclusion criteria were animal or in vitro studies, patients <18 years, non-stroke participants, biomarkers measured prior to event, and other study designs (crossover studies, cross-sectional studies, editorials, case reports, review articles, or duplicate studies).
Without language restrictions, we searched Ovid Medline (1/1/1946–10/1/2019) and Embase (1/1/1970–10/1/2019) using a combination of medical subject headings and free text search terms (web-supplement). Abstract screening and full text review were performed independently by 2 reviewers (JM, EO) with disagreements regarding eligibility resolved by consensus with the participation of a third reviewer (PK).
Assessment of study quality
The Quality In Prognosis Studies (QUIPS) tool was used to assess the risk of bias of included studies and was adjudicated by 2 authors (JM, EO).14 QUIPS evaluates six domains of potential bias in prognostic research: (i) study participation; (ii) study attrition; (iii) prognostic factor measurement; (iv) confounder measurement; (v) outcome measurement; (vi) analysis/reporting. Additionally we ascertained whether 9 specific measures of methodological quality were present: (i) reference to the presence of any study protocol; (ii) prior publication of a biomarker protocol; (iii) assay specified; (iv) blinded biomarker measurement; (v) blinded outcome assessment; (vi) outcome explicitly defined; (vii) screening for infection/inflammatory conditions; (viii/ix) measurement and adjustment of cardiovascular risk factors (age, smoking, diabetes mellitus, lipids/statin therapy, blood pressure, obesity).
Data extraction
Details of the data extraction protocol are provided in the web-supplement. Briefly, data extraction was performed by 2 reviewers (JM, EO) using a standardized template. All measures of association between inflammatory markers and outcome were recorded (eg. odds ratio (OR), hazard ratio (HR), mean/median differences between marker levels in groups with/without the specified outcome event). Where multiple effect estimates were provided, the most adjusted measure of effect was chosen for the final analysis.
Statistical analyses
Baseline study characteristics were summarized using mean/SD or median/interquartile range (IQR). Forest plots were used to visually summarize study effect estimates and their corresponding 95% CIs, for each biomarker and each pre-specified outcome. A pooled meta-analysis using a random effects model15 was restricted to studies reporting homogenous effect measures in accordance with best-practice recommendations.16,17 Between-study heterogeneity was assessed by the I2 statistic.18 We investigated for small study effects and publication bias using contour-enhanced funnel plots and tested for the presence of funnel plot asymmetry using Egger’s test where appropriate.19,20 STATA 15.0 (StataCorp, College Station, TX) software was used for statistical analyses. A p value <0.05 was considered significant.
Results
Search results
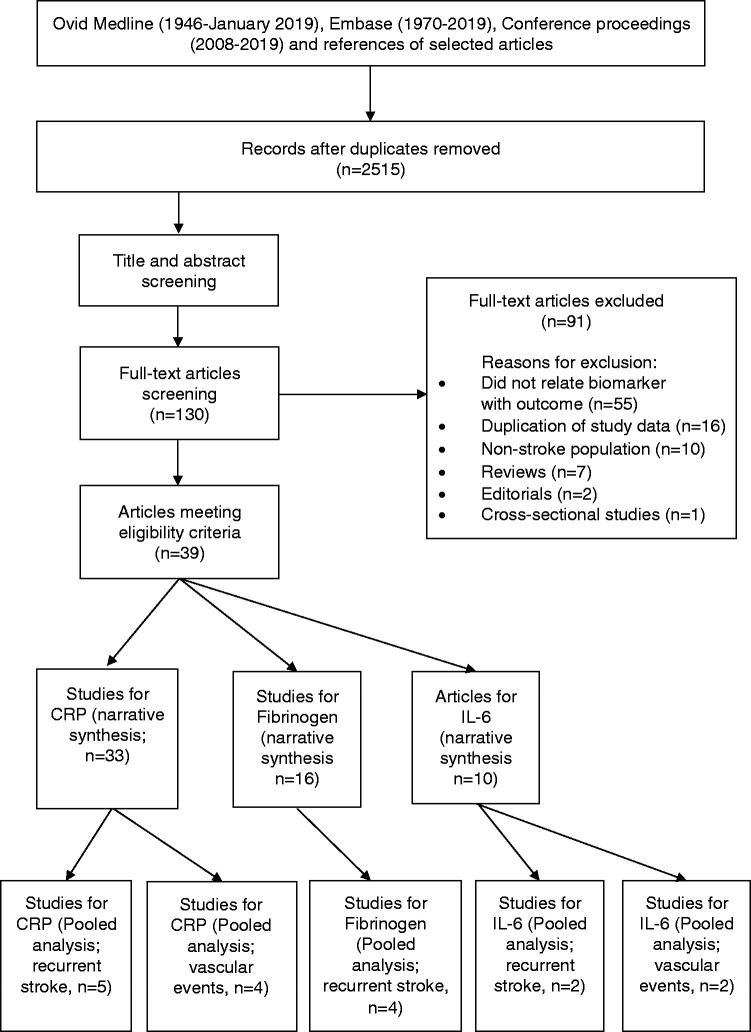
The original search identified 2,515 non-duplicate records. After title and abstract screening, 130 studies were selected for full text review. Thirty-nine articles met eligibility criteria (37 individual study cohorts). There were 33 reports from 30 studies for CRP,21–53 14 reports from 16 studies for fibrinogen21,36–39,42–44,47,54–58 and 10 studies for IL-6 (Figure 1).21,24,37–40,43,47,48,59
Figure 1.
PRISMA Flowchart for the selection of eligible articles.
Quality assessment
Using the QUIPS tool, study attrition, study confounding and outcome assessment were the 3 most frequently identified domains for risk of bias [Supplementary Figure 1(a-c)]. The 9 studies included in the pooled analysis had a lower risk of bias across most domains when compared with studies in the narrative synthesis. Only one study was deemed a high risk of bias in 2 domains [Supplementary Table 1]. The methodological quality assessment found that most studies did not publish a study protocol, screen for infectious/inflammatory conditions, specify blinded outcome adjudication or measure/adjust for all cardiovascular risk factors [Supplementary Figure 2(a-c)].
CRP and risk of recurrent stroke and vascular events
The search identified 30 studies for CRP with 25,363 patients (Supplementary Table 2). Where stated, CRP measurement was in the acute phase (0–14 days after the index event) in 61% of studies and in the subacute or convalescent phase in 39%. 12 studies explicitly stated that participants were screened for infectious/inflammatory conditions. There was marked variability in the definition of CRP exposure across studies, including risk analysed per increase in standard deviation, per tertile/quartile/decile, per measured unit (mg/dL) or by arbitrary cut-points on log-transformed and untransformed scales [Supplementary Figure 3(a-b), Supplementary Table 3]. Only 6/30 studies adjusted for all pre-specified vascular risk factors.
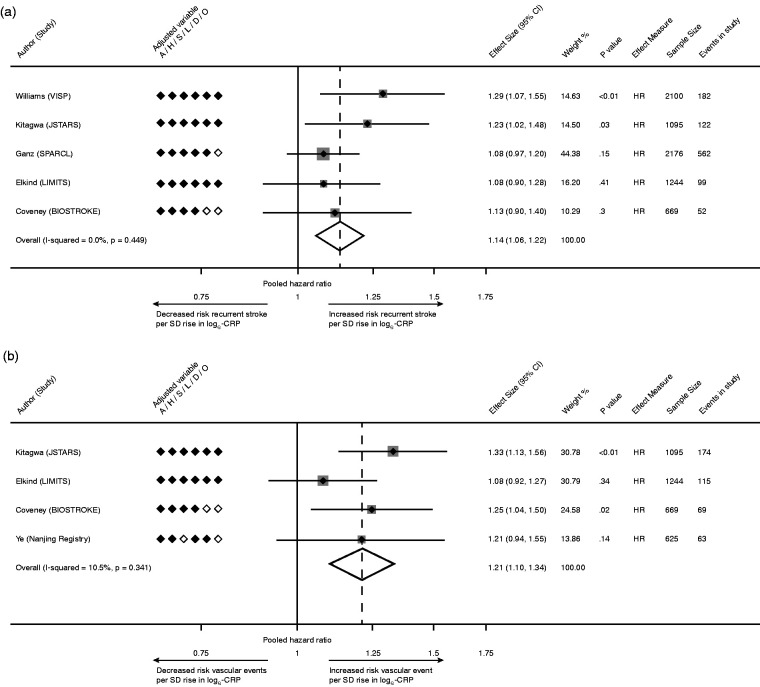
The association of CRP with recurrent stroke was reported in 26 studies, of which 12 (46%) described a positive association. A meta-analysis was limited to 5 studies (7,284 patients) which uniformly analyzed the risk of recurrent stroke per 1-SD rise in loge-CRP.28,29,32,44,48 Using a random-effects model, the pooled HR of recurrent stroke per 1-SD rise in loge-CRP was 1.14 [95% CI 1.06–1.22, p < 0.01; Figure 2(a)], with low heterogeneity (I2 = 0%).
Figure 2.
(a) Pooled analysis of studies reporting risk of recurrent stroke per 1-SD rise in loge-CRP; (b) Pooled estimate for risk of recurrent MVEs per 1-SD rise in loge-CRP. Legend: A, age; H, hypertension; S, smoking; L, hyperlipidemia; D, diabetes mellitus; O, obesity; HR, hazard ratio.
The association of CRP with recurrent MVEs was reported in 17 studies, of which 10 (59%) reported a positive association. Due to heterogeneity in CRP measures between studies, meta-analysis was confined to 4 studies (3,633 patients) that reported risk per SD rise in loge-CRP.28,32,45,48 The HR of MVEs per 1-SD increase in loge-CRP was 1.21 [95% CI 1.10–1.34, p < 0.01; Figure 2(b)], with low heterogeneity (I2 = 10.5%).
Fibrinogen and risk of recurrent stroke and vascular events
There were 16 studies for fibrinogen (n = 12,961 patients, Supplementary Table 4). Thirty-eight percent of studies measured fibrinogen in the acute phase after the index event and in the subacute or convalescent phase in the remainder. Just 2 studies screened for infectious/inflammatory conditions. There was also a marked variability in the definition of fibrinogen exposure and measures of association reported across studies [Supplementary Figure 4(a-b), Supplementary Table 5]). Only 1 study fully adjusted for all vascular risk factors.
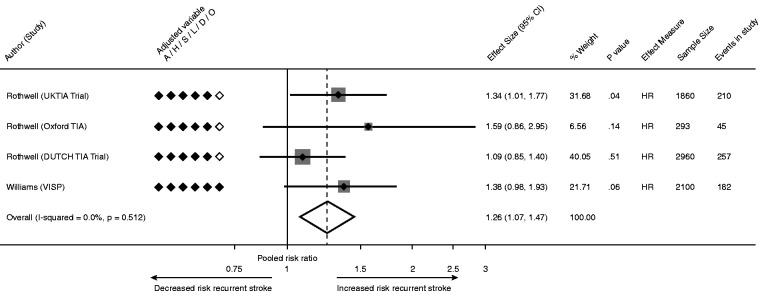
The association between fibrinogen and recurrent stroke was reported in 11 studies, of which 2 reported a positive association. A pooled analysis was restricted to 4 studies (n = 6,995) which reported risk of supra-median versus sub-median fibrinogen44,57 The pooled HR of recurrent stroke associated with elevated fibrinogen was 1.26 (95% CI 1.07–1.47, p < 0.01; Figure 3), without statistical heterogeneity (I2 = 0%).
Figure 3.
Pooled estimate for risk of recurrent stroke for supra-median compared with sub-median fibrinogen level.
Five of the 10 studies investigating fibrinogen and risk of MVEs reported a positive association. A meta-analysis was possible for 3 comparable studies which reported HRs for supra- versus sub-median fibrinogen. This analysis was previously published by Rothwell et al. and reported increased risk of MVEs with elevated fibrinogen (HR 1.31, 95% CI 1.15–1.49, p < 0.01, I2 = 0%).57 We independently repeated this analysis and found identical results.
IL-6 And risk of recurrent stroke and vascular events
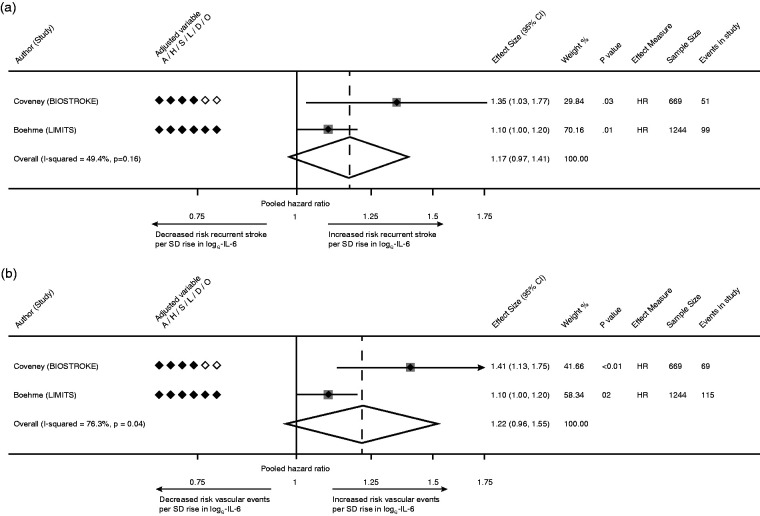
Ten studies were identified for IL-6 (n = 7,934, Supplementary Table 6) with marked variability in definitions of IL-6 exposure, timing of phlebotomy, screening for infection and measures of association [Supplementary Figure 5(a-b), Supplementary Table 7]. Three of six studies reported a positive association with recurrent stroke. Only 1 study fully adjusted for all vascular risk factors. Due to varying methodology, only two comparable studies (n = 1,913) were meta-analysed which analysed risk per 1-SD rise in loge-IL-6.48,59. A non-significant statistical trend was identified for the association between IL-6 and recurrent stroke (HR per 1-SD increase 1.17 [95% CI 0.97–1.41, p = 0.10; Figure 4(a)] with moderate heterogeneity (I2 = 49.4%).
Figure 4.
(a) Pooled estimate for risk of recurrent stroke per 1-SD rise in loge-IL-6; (b) Pooled estimate for risk of recurrent MVEs per 1-SD rise in loge-IL-6.
Of 7 studies which reported the association of IL-6 with MVEs, 5 reported a positive association. Meta-analysis was again restricted to 2 comparable studies (n = 1,913).48,59 A non-significant trend was identified for the association of IL-6 with MVEs [pooled HR 1.22, 95% CI 0.96–1.55, p = 0.10; Figure 4(b)], with considerable statistical heterogeneity (I2 = 76.3%).
Small study effects
There was evidence of funnel-plot asymmetry for outcomes of recurrent stroke (Egger’s p < 0.01) and MVEs (Egger’s p < 0.01) for CRP, indicating possible publication bias. Asymmetry was also apparent on funnel-plot inspection of fibrinogen studies, but statistical assessment was not possible due to few available studies [Supplementary Figure 6(a-d)]. Funnel-plot analysis was not possible for IL-6 due to the small number of studies.
Discussion
Atherosclerotic inflammation has emerged as a therapeutic target for prevention of coronary events and stroke. The central inflammatory pathway in atherosclerotic plaque development involves upstream interleukin-1β (IL-1β) activation by the NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome in response to cholesterol crystal deposition and other pro-inflammatory stimuli. The pleiotropic cytokine IL-6 is expressed by T-cells and monocyte-macrophages in response to IL-1β, and has multiple pro-atherogenic and pro-inflammatory effects including hepatocyte production of CRP and fibrinogen.60,61 To inform the design of randomised trials of anti-inflammatory therapies for secondary prevention after stroke, robust data on inflammatory biomarkers are needed.
In this context, we report the first systematic review and meta-analysis to investigate the association of IL-6, fibrinogen and CRP and vascular recurrence after stroke. Our study provides new data in several ways: First, we found important variability in methodological aspects such as inclusion of patients with severe stroke or other confounding pro-inflammatory diseases, adjustment for confounders, and definition of exposure variables. This variability limits the interpretation of the available evidence and indicates that greater standardisation should be a priority in the design and reporting of future studies.16 Second, on pooled analysis of the sub-set of comparable studies we found that each SD increase in loge-CRP was associated with a one-fifth increase in risk of future major vascular events in stroke survivors. These findings were consistent across studies, without evidence of heterogeneity. Based on individual-participant data available, 1-SD rise in loge-CRP corresponds to an approximate four-fold difference in mg/L on the original scale of CRP measurement. Although caution is needed due to the unavoidable selection of a sub-group of available studies, the results are biologically-plausible and consistent with those reported for patients with coronary disease.6
Third, similar results were found for fibrinogen, where pooled risks were increased by 26% (recurrent stroke) and 31% (all vascular events) when patients with supra- versus sub-median levels were compared. This finding is consistent with other data showing an association of fibrinogen with risk of first stroke and coronary events.7 Fourth, although only 2 studies could be pooled, analysis for IL-6 also showed non-significant but consistent directions of association for both recurrent stroke and MVEs. Overall, the available data suggested a consistent pattern of increased vascular risk in stroke patients with elevated blood inflammatory markers.
Our findings add to other evidence suggesting that atherosclerotic inflammation is an independent risk factor for ischaemic vascular events. In epidemiological studies, CRP, fibrinogen and MCP-1 are associated with first stroke.6–8 Genetic variants predisposing to higher MCP-1 levels are associated with increased stroke risk, whilst genetic predisposition to reduced IL-6 receptor signalling is protective against stroke and coronary events.8,9,62 In PET-imaging studies, carotid plaque 18-fluorodeoxyglucose (18F-FDG) uptake predicts early recurrent stroke, independent of stenosis severity.5 In randomised placebo-controlled trials, IL-1β inhibition with canakinumab and tubulin/NLRP3 inhibition with colchicine reduce recurrent events in patients with coronary disease.10–12
Strengths of our study are its comprehensiveness, use of recommended methods for systematic reviews of observational studies, and assessment of key inflammatory biomarkers which are most likely to have utility in randomised trials and clinical practice. Due to variability in the definition of exposure variables, we deliberately restricted pooled analysis to a sub-set of comparable studies, which allows valid interpretation of these results.
We acknowledge several limitations. We cannot exclude the possibility of selection bias in studies included for pooled analyses. Because individual patient data were unavailable, pooled risk estimates were not fully adjusted for all possible confounding variables. As with all systematic reviews, the methodological quality of included studies and possibility of publication bias need to be considered in the interpretation of our findings. Our selection of studies for pooled analysis based on exposure variables defined as continuous measures was not pre-specified. However, we believe that this approach is appropriate, as analysis on continuous scales is recommended in prognostic research.16
Conclusion
Our review provides new evidence suggesting that inflammation is associated with increased risk of future vascular events in stroke patients. An individual-participant data meta-analysis is needed to determine whether an association remains after adjustment for residual confounding, and to address other knowledge gaps such as optimal choice of biomarker, relationship with stroke subtypes, optimal timing of measurement, and impact of important factors such as infarct volume and stroke severity. Our findings support the rationale for randomised trials of anti-inflammatory therapies after stroke. One such trial, CONVINCE (ClinicalTrials.gov Identifier: NCT02898610) is currently investigating low-dose colchicine after ischaemic stroke or TIA.
Supplemental Material
Supplemental material, sj-zip-1-eso-10.1177_2396987320984003 for Interleukin-6, C-reactive protein, fibrinogen, and risk of recurrence after ischaemic stroke: Systematic review and meta-analysis by JJ McCabe E O’Reilly, S Coveney R Collins, L Healy, J McManus, R Mulcahy, B Moynihan, T Cassidy, F Hsu, B Worrall in European Stroke Journal
Acknowledgements
Stroke Clinical Trials Network Ireland (SCTNI) investigators.
Data availability: Data are available on reasonable request.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: PJK – funded from Health Research Board Ireland and Irish Heart Foundation.
Ethical approval: The data included in this analysis was extracted from previously published work. Therefore, no additional ethical approval or consent was required.
Guarantor: PJK.
Contributorship: JJM/EOR/PJK – literature search. JJM/PJK/EOR/SC/RC/LH/JM/RM/BM/TC/SM/MOD contributed to study conception, protocol design and drafting of the manuscript. BW/FH provided additional unpublished data.
ORCID iDs: JJ McCabe https://orcid.org/0000-0003-2029-1303
S Coveney https://orcid.org/0000-0003-1442-6320
B Worrall https://orcid.org/0000-0001-9386-4091
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Boulanger M, Bejot Y, Rothwell PM, et al. Long-Term risk of myocardial infarction compared to recurrent stroke after transient ischemic attack and ischemic stroke: systematic review and meta-analysis. J Am Heart Assoc 2018; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redgrave JNE, Lovett JK, Gallagher PJ, et al. Histological assessment of 526 symptomatic carotid plaques in relation to the nature and timing of ischemic symptoms – The oxford plaque study. Circulation 2006; 113: 2320–2328. [DOI] [PubMed] [Google Scholar]
- 3.Marnane M, Prendeville S, McDonnell C, et al. Plaque inflammation and unstable morphology are associated with early stroke recurrence in symptomatic carotid stenosis. Stroke 2014; 45: 801–806. [DOI] [PubMed] [Google Scholar]
- 4.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 2002; 105: 1135–1143. [DOI] [PubMed] [Google Scholar]
- 5.Kelly PJ, Camps-Renom P, Giannotti N, et al. Carotid plaque inflammation imaged by (18)F-Fluorodeoxyglucose positron emission tomography and risk of early recurrent stroke. Stroke 2019; 50: 1766–1773. [DOI] [PubMed] [Google Scholar]
- 6.Emerging Risk Factors C, Kaptoge S, Di Angelantonio E, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant Meta-analysis. Lancet 2010; 375: 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danesh J, Lewington S, Thompson SG, et al.; Fibrinogen Studies Collaboration. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA 2005; 294: 1799–1809. [DOI] [PubMed] [Google Scholar]
- 8.Georgakis MK, Gill D, Rannikmae K, et al. Genetically determined levels of circulating cytokines and risk of stroke. Circulation 2019; 139: 256–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Georgakis MK, Malik R, Gill DK, et al. Interleukin-6 signaling effects on ischemic stroke and other cardiovascular outcomes: a Mendelian randomization study. medRxiv 2019; 13: 19007682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nidorf SM, Eikelboom JW, Budgeon CA, et al. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol 2013; 61: 404–410. [DOI] [PubMed] [Google Scholar]
- 11.Tardif JC, Kouz S, Waters DD, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 2019; 381: 2497–2505. [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM, Everett BM, Thuren T, et al.; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377: 1119–1131. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, et al.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 2006; 144: 427–437. [DOI] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 16.Riley RD, Hayden JA, Steyerberg EW, et al.; PROGRESS Group. Prognosis research strategy (PROGRESS) 2: prognostic factor research. PLoS Med 2013; 10: e1001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riley RD, Moons KGM, Snell KIE, et al. A guide to systematic review and meta-analysis of prognostic factor studies. Bmj 2019; 364: k4597. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 19.Peters JL, Sutton AJ, Jones DR, et al. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol 2008; 61: 991–996. [DOI] [PubMed] [Google Scholar]
- 20.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Bmj 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beamer NB, Coull BM, Clark WM, et al. Persistent inflammatory response in stroke survivors. Neurology 1998; 50: 1722–1728. [DOI] [PubMed] [Google Scholar]
- 22.Arenillas JF, Álvarez-Sabín J, Molina CA, et al. C-reactive protein predicts further ischemic events in first-ever transient ischemic attack or stroke patients with intracranial large-artery occlusive disease. Stroke 2003; 34: 2463–2468. [DOI] [PubMed] [Google Scholar]
- 23.Campbell DJ, Woodward M, Chalmers JP, et al. Prediction of myocardial infarction by N-terminal-pro-B-type natriuretic peptide, C-reactive protein, and renin in subjects with cerebrovascular disease. Circulation 2005; 112: 110–116. [DOI] [PubMed] [Google Scholar]
- 24.Castillo J, Alvarez-Sabin J, Martinez-Vila E, et al.; MITICO Study Investigators. Inflammation markers and prediction of post-stroke vascular disease recurrence: the MITICO study. J Neurol 2009; 256: 217–224. [DOI] [PubMed] [Google Scholar]
- 25.Corso G, Bottacchi E, Brusa A, et al. Is there a prognostic role for C-reactive protein in ischemic stroke? Acta Neurol Scand 2010; 122: 209–216. [DOI] [PubMed] [Google Scholar]
- 26.Corso G, Bottacchi E, Brusa A, et al. Blood C-reactive protein concentration with ABCD(2) is a better prognostic tool than ABCD(2) alone. Cerebrovasc Dis 2011; 32: 97–105. [DOI] [PubMed] [Google Scholar]
- 27.Elkind MS, Tai W, Coates K, et al. High-sensitivity C-reactive protein, lipoprotein-associated phospholipase A2, and outcome after ischemic stroke. Arch Intern Med 2006; 166: 2073–2080. [DOI] [PubMed] [Google Scholar]
- 28.Elkind MS, Luna JM, McClure LA, et al.; LIMITS Investigators. C-reactive protein as a prognostic marker after lacunar stroke: levels of inflammatory markers in the treatment of stroke study. Stroke 2014; 45: 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ganz P, Amarenco P, Goldstein LB, et al. Association of osteopontin, neopterin, and myeloperoxidase with stroke risk in patients with prior stroke or transient ischemic attacks: results of an analysis of 13 biomarkers from the stroke prevention by aggressive reduction in cholesterol levels trial. Stroke 2017; 48: 3223–3231. [DOI] [PubMed] [Google Scholar]
- 30.Kuwashiro T, Sugimori H, Ago T, et al. Risk factors predisposing to stroke recurrence within one year of non-cardioembolic stroke onset: the Fukuoka stroke registry. Cerebrovasc Dis 2012; 33: 141–149. [DOI] [PubMed] [Google Scholar]
- 31.Kuwashiro T, Sugimori H, Ago T, et al.; FSR Investigators (see appendix). Predictive role of C reactive protein in stroke recurrence after cardioembolic stroke: the Fukuoka stroke registry. BMJ Open 2013; 3: e003678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitagawa K, Hosomi N, Nagai Y, J-STARS Investigators et al. Reduction in high-sensitivity C-reactive protein levels in patients with ischemic stroke by statin treatment: Hs-CRP Sub-Study in J-STARS. J Atheroscler Thromb 2017; 24: 1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krarup LH, Sandset EC, Sandset PM, et al. D-dimer levels and stroke progression in patients with acute ischemic stroke and atrial fibrillation. Acta Neurol Scand 2011; 124: 40–44. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Zhao X, Meng X, et al.; on behalf of the CHANCE Investigators. High-sensitive C-reactive protein predicts recurrent stroke and poor functional outcome: subanalysis of the clopidogrel in high-risk patients with acute nondisabling cerebrovascular events trial. Stroke 2016; 47: 2025–2030. [DOI] [PubMed] [Google Scholar]
- 35.Matsuo R, Ago T, Hata J, et al.; on behalf of the Fukuoka Stroke Registry Investigators. Plasma C-Reactive protein and clinical outcomes after acute ischemic stroke: a prospective observational study. PLoS One 2016; 11: e0156790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purroy F, Montaner J, Molina CA, et al. C-reactive protein predicts further ischemic events in transient ischemic attack patients. Acta Neurol Scand 2007; 115: 60–66. [DOI] [PubMed] [Google Scholar]
- 37.Purroy F, Suarez-Luis I, Cambray S, et al. The determination of copeptin levels helps management decisions among transient ischaemic attack patients. Acta Neurol Scand 2016; 134: 140–147. [DOI] [PubMed] [Google Scholar]
- 38.Segal HC, Burgess AI, Poole DL, et al. Population-based study of blood biomarkers in prediction of subacute recurrent stroke. Stroke 2014; 45: 2912–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Selvarajah JR, Smith CJ, Hulme S, et al. Does inflammation predispose to recurrent vascular events after recent transient ischaemic attack and minor stroke? The North West of England transient ischaemic attack and minor stroke (NORTHSTAR) study. Int J Stroke 2011; 6: 187–194. [DOI] [PubMed] [Google Scholar]
- 40.Silvestri A, Vitale C, Ferretti F, et al. Plasma levels of inflammatory C-reactive protein and interleukin-6 predict outcome in elderly patients with stroke. J Am Geriatr Soc 2004; 52: 1586–1587. [DOI] [PubMed] [Google Scholar]
- 41.Shibazaki K, Kimura K, Aoki J, et al. Brain natriuretic peptide level on admission predicts recurrent stroke after discharge in stroke survivors with atrial fibrillation. Clin Neurol Neurosurg 2014; 127: 25–29. [DOI] [PubMed] [Google Scholar]
- 42.Uehara T, Ohara T, Minematsu K, et al. Predictors of stroke events in patients with transient ischemic attack attributable to intracranial stenotic lesions. Intern Med 2018; 57: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whiteley W, Jackson C, Lewis S, et al. Association of circulating inflammatory markers with recurrent vascular events after stroke: a prospective cohort study. Stroke 2011; 42: 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams SR, Hsu FC, Keene KL, et al. Shared genetic susceptibility of vascular-related biomarkers with ischemic and recurrent stroke. Neurology 2016; 86: 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye Z, Zhang Z, Zhang H, et al. Prognostic value of C-reactive protein and homocysteine in large-artery atherosclerotic stroke: a prospective observational study. J Stroke Cerebrovasc Dis 2017; 26: 618–626. [DOI] [PubMed] [Google Scholar]
- 46.Zhang YB, Yin Z, Han X, et al. Association of circulating high-sensitivity C-reactive protein with late recurrence after ischemic stroke. Neuroreport 2017; 28: 598–603. [DOI] [PubMed] [Google Scholar]
- 47.Welsh P, Lowe GD, Chalmers J, et al. Associations of proinflammatory cytokines with the risk of recurrent stroke. Stroke 2008; 39: 2226–2230. [DOI] [PubMed] [Google Scholar]
- 48.Coveney S, Murphy S, Belton O, et al. Interleukin-6 independently predicts late fatality and poor functional outcome after transient ischaemic attack (TIA) and non severe stroke. Eur Stroke J 2018; 3: 366. [Google Scholar]
- 49.Hong JB, Endres M, Siegerink B, et al. High-sensitivity c-reactive protein in acute stroke patients and vascular risk in the first year: Cohort study and systematic review. Eur Stroke J 2016; 1: 255.31008286 [Google Scholar]
- 50.Huang Y, Jing J, Zhao XQ, et al. High-sensitivity C-reactive protein is a strong risk factor for death after acute ischemic stroke among chinese. CNS Neurosci Ther 2012; 18: 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Öztürk M, Yüzbaşioğlu Y, Akinci E, et al. An evaluation of ABCD2 scores, atrial fibrillation, serum CRP, fibrinogen and D-dimer levels as diagnostic predictors for stroke in patients admitted to emergency department with the diagnosis of transient ischemic attack. J Neurol Sci 2016; 33: 473–481. [Google Scholar]
- 52.Penko M, Hojs Fabjan T, Bevc S, et al. A prospective study about impact of renal dysfunction and morbidity and mortality on cardiovascular events after ischemic stroke. Cardiol J 2014; 21: 163–169. [DOI] [PubMed] [Google Scholar]
- 53.Ueno Y, Yamashiro K, Tanaka R, et al. Factors influencing recurrent stroke: TEE study with longitudinal observation in cryptogenic stroke. Stroke 2016; 47: 2714–2721.27703086 [Google Scholar]
- 54.Bruno A, McConnell JP, Cohen SN, et al. Plasma thrombosis markers following cerebral infarction in African Americans. Thromb Res 2005; 115: 73–77. [DOI] [PubMed] [Google Scholar]
- 55.Pedersen A, Redfors P, Lundberg L, et al. Haemostatic biomarkers are associated with long-term recurrent vascular events after ischaemic stroke. Thromb Haemost 2016; 116: 537–543. [DOI] [PubMed] [Google Scholar]
- 56.Resch KL, Ernst E, Matrai A, et al. Fibrinogen and viscosity as risk factors for subsequent cardiovascular events in stroke survivors. Ann Intern Med 1992; 117: 371–375. [DOI] [PubMed] [Google Scholar]
- 57.Rothwell PM, Howard SC, Power DA, et al. Fibrinogen concentration and risk of ischemic stroke and acute coronary events in 5113 patients with transient ischemic attack and minor ischemic stroke. Stroke 2004; 35: 2300–2305. [DOI] [PubMed] [Google Scholar]
- 58.Shibazaki K, Kimura K, Aoki J, et al. Plasma brain natriuretic peptide as a predictive marker of early recurrent stroke in cardioembolic stroke patients. J Stroke Cerebrovasc Dis 2014; 23: 2635–2640. [DOI] [PubMed] [Google Scholar]
- 59.Boehme AK, McClure LA, Zhang Y, et al. Inflammatory markers and outcomes after lacunar stroke: levels of inflammatory markers in treatment of stroke study. Stroke 2016; 47: 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kelly PJ, Murphy S, Coveney S, et al. Anti-inflammatory approaches to ischaemic stroke prevention. J Neurol Neurosurg Psychiatry 2018; 89: 211–218. [DOI] [PubMed] [Google Scholar]
- 61.Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ Res 2016; 118: 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sarwar N, Butterworth AS, Freitag DF, et al.; Collaboration IRGCERF. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet 2012; 379: 1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-zip-1-eso-10.1177_2396987320984003 for Interleukin-6, C-reactive protein, fibrinogen, and risk of recurrence after ischaemic stroke: Systematic review and meta-analysis by JJ McCabe E O’Reilly, S Coveney R Collins, L Healy, J McManus, R Mulcahy, B Moynihan, T Cassidy, F Hsu, B Worrall in European Stroke Journal