Why was the EN-BIRTH study needed?
Unacceptably, 2.4 million newborns were estimated to have died during their first 28 days in 2019 [1]. Additionally at least 2 million babies each year were stillborn in the last 3 months of pregnancy [2], many during labour and many to the 0.3 million mothers who die from maternal causes each year [3]. Millions more babies were born too soon, at increased risk of long-term disabilities [4]. In 2014, the Every Newborn Action Plan (ENAP) [5, 6] was endorsed by 194 Member States, including a commitment to end preventable newborn deaths and stillbirths. The first ever global target for neonatal mortality reduction was included in Sustainable Development Goal (SDG) 3 [7]. To attain universal health coverage and meet SDG 3 by 2030, countries need to scale up evidence-based interventions, including for newborn health. Hence timely and high-quality data on outcomes and coverage are crucial, especially through national health information systems. During pandemics, stillbirths and neonatal deaths may be increased [8], further underlining the need for data through routine systems.
Core indicators to track progress in maternal and newborn health were prioritized through evidence review, and an inclusive consultation process undertaken through ENAP [9, 10]. In high burden settings, the majority of comparable data for these indicators are currently collected through population-based surveys, and no rigorous validation studies have been undertaken until now for facility-based maternal and newborn indicators in routine health information systems [10]. A multi-partner measurement improvement roadmap [11] was developed for 2015–2020 to improve the ENAP core indicator definitions and to test their measurement validity – including capturing care for newborns at risk or with complications – and inform feasibility of measurement. This roadmap highlighted a major gap for measurement of coverage and quality of care, including service readiness.
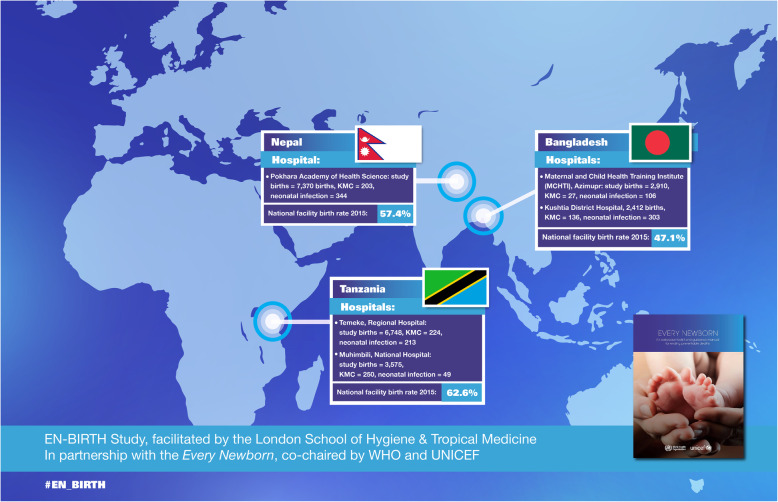
The Every Newborn - Birth Indicators Research Tracking in Hospitals (EN-BIRTH) study is directly linked to the ENAP measurement improvement roadmap, and ultimately SDG3. The study’s primary aim was to validate, by comparison to direct clinical observation as the gold standard, data from routine facility registers and women’s survey report for capturing facility-based coverage and quality of care [12]. EN-BIRTH was conducted in five hospitals providing comprehensive emergency obstetric and newborn care in three high-burden mortality countries: Tanzania, Bangladesh, and Nepal (Fig. 1). The multi-country EN-BIRTH team observed 23,471 births and 840 kangaroo mother care (KMC) mother-baby pairs, in addition to collecting information on 1015 admissions for neonatal infection. The three country research teams represent ENAP priority countries from sub-Saharan Africa and south Asia. The multi-country research team actively co-designed the study, facilitated by a team at the London School of Hygiene & Tropical Medicine (LSHTM) and funded by Children’s Investment Fund Foundation (CIFF). The large quantitative dataset and standardised approach to qualitative data collection enabled the synthesis of barriers/enablers to collection and use of data in routine registers.
Fig. 1.
EN-BIRTH five study sites in three countries. National facility birth rates are for 2013-2018 [13]
What is new and what have we learned?
The EN-BIRTH study provides many important findings to advance measurement to drive change. Here we focus on the 14 papers in this supplement (Fig. 2). The overall validation results from EN-BIRTH are published separately [14]. This supplement starts with three papers on measurement systems covering: EN-BIRTH electronic data collection [15], survey-report for validity of 33 indicators [16], and barriers/enablers to recording in routine registers [17]. Subsequent papers detail findings for the following maternal and newborn coverage indicators: uterotonics to prevent postpartum haemorrhage [18], immediate newborn care including breastfeeding practices [19], chlorhexidine for umbilical cord care [20], neonatal resuscitation [21], KMC [22], neonatal infection antibiotic management [23]. Two papers assess validity and data quality for the outcomes of birthweight [24] and stillbirth [25]. Measurement of respectful maternal and newborn care is assessed in one site (Nepal) [26]. Processes and perceptions for birthweight [27] and birth registration measurement [28] are examined in the Tanzanian sites. These papers all outline actions for improving measurement now and proposing what research is needed next.
Fig. 2.
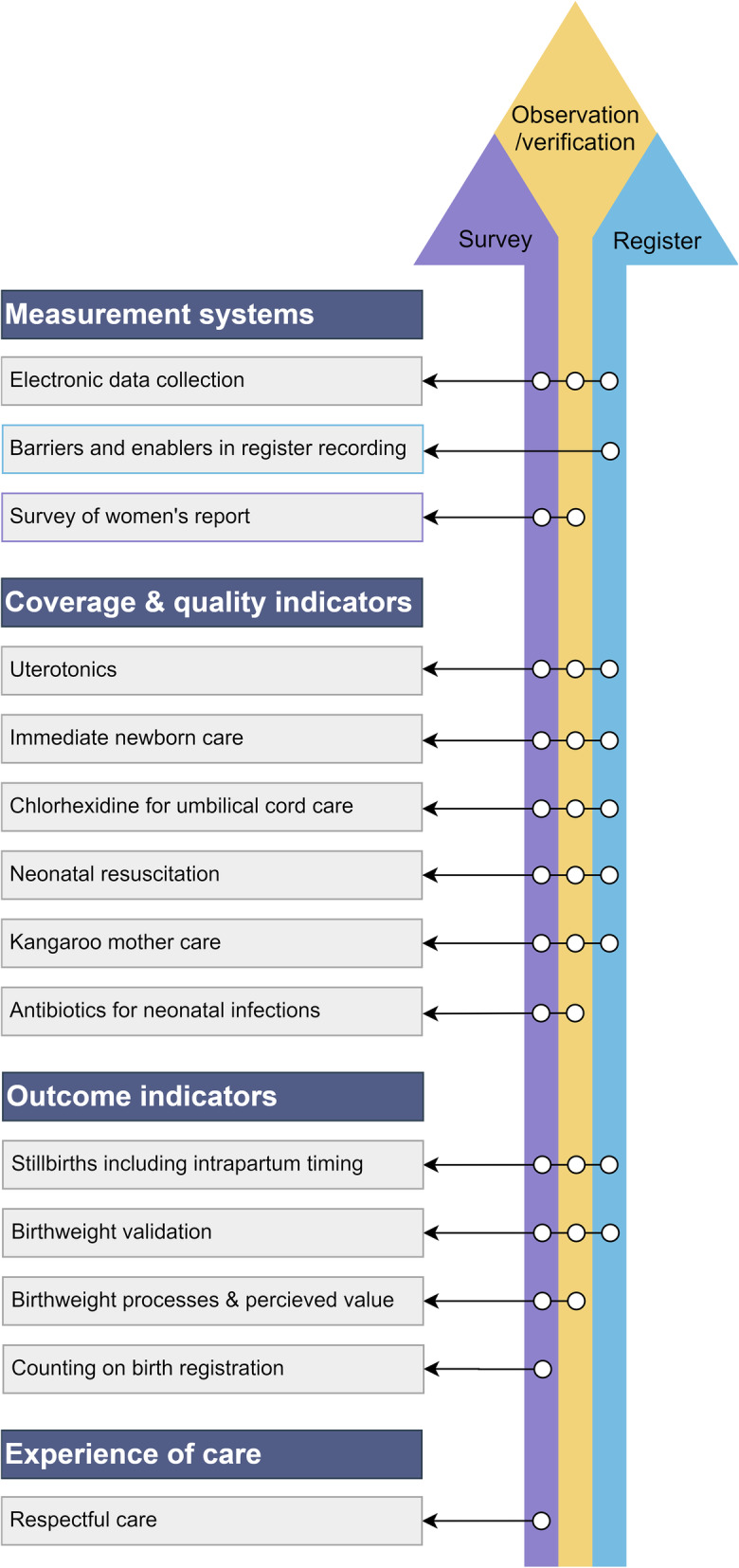
Overview of the three data types in EN-BIRTH study and the 14 papers in the supplement. Key findings for supplement papers are presented in Additional file 1
Women’s survey-report performed fairly well for birthweight, although with more heaping than in register data [24]. EN-BIRTH shows for the first time that such surveys may be a useful tool for capturing information on contact indicators, such as admission to a neonatal unit [23] or KMC ward. However, survey questions have low accuracy for most maternal and newborn clinical interventions. For example, the results on measuring either the numerator or denominator for antibiotic use for neonatal infections are consistent with findings regarding low survey validity for antibiotic treatment for childhood pneumonia [29]. Clinical interventions that include timing pose additional challenges in survey data collection platforms. For instance, early initiation of breastfeeding within 1 h of birth was overestimated by women's survey report, compared to time-stamped observer data [19]. More research is needed on the validity of survey questions for clinical interventions, including less focus on a rigid time schedule.
Routine hospital registers had high validity for most clinical interventions assessed in at least one hospital, provided the register design included the indicator. Registers performed particularly well for indicators regarding uterotonics for prevention of postpartum hemorrhage, chlorhexidine cord application, and have potential for neonatal bag-and-mask resuscitation [18, 20, 21]. Design of registers is critical for improving reporting accuracy, even the labour ward register designs varied across the three countries. There is much work to be done to standardise register content and link registers with individual patient case notes to reduce the number of times health workers are required to record duplicate data. To increase the data availability at the national level, registers will first need to be standarised to capture a parsimonious indicator list, including the linked data elements for numerator and denominator of each indicator. Second, these data need to be linked with other hospital documentation, including the flow into digital platforms. Implementation of the standardised registers need local ownership, to increase the likelihood of uptake and, importantly, local use of routine data in improving care and monitoring.
Quality of care had many gaps compared to global standards, notably regarding timing of care. For example, although provision of uterotonics to prevent postpartum hemorrhage was universally high across all five hospitals, the observational data showed that overall only 16% of women received uterotonics within 1 min after birth [18]. Regarding neonatal resuscitation, most non-breathing newborns were observed to receive bag-mask-ventilation, but overall only 1% within the recommended 1 min after birth [21]. Whilst nearly all babies were weighed within 1 h of birth (97%), only 16% were weighed using digital scales [24]. Most stillbirths were weighed, apart from one site [25]. Antibiotic stewardship was also a serious issue across the study sites. Overall only 11% of newborn inpatients had a blood culture; even fewer had a lumbar puncture (< 1%) and few newborns received the recommended antibiotics for the optimum duration. For KMC, ward registers accurately captured admission to care (a service contact measure), yet there were gaps identified in quality of care, especially duration and feeding support [22]. These findings indicate a need for further research to determine the underlying causes of the poor quality of care so that remedial action can be taken.
A novel finding, based on the large number of women who had a caesarean section (6698) in this study, is the effect of mode of birth on other care practices and register or survey report measurement. Given the rising global rates of caesarean sections, care practices and measurement implications require more study.
What next for improving and using data on coverage and quality?
EN-BIRTH is the first multi-site, facility-based study validating measures in routine register data for maternal and newborn care and in women’s survey-report for newborns with complications. Both data sources have value, yet both have limitations. Women’s survey-report can be used effectively for collecting certain information, notably service contact points – as is already done for antenatal care, institutional birth, and postnatal care. EN-BIRTH results show high validity for survey questions on admission both to KMC ward and newborn care ward. These questions performed well, but further testing is required among those whose newborns were not admitted; longer recall (2-5 years) and survey sample size also need to be considered [16]. EN-BIRTH clearly adds to findings from previous research that surveys are not an appropriate tool for capturing valid information on clinical interventions provided around the time of birth, and that more work is needed to refine survey indicators based on timing of care, such as early initiation of breastfeeding [30, 31]. Surveys may be useful for measuring experience of care, but there are notable challenges in women’s ability to report their experience of care, especially when asking questions in or near the facility [26, 32].
Registers in labour wards, operating theatres, KMC wards and newborn care wards have tremendous potential to track facility-based maternal and newborn interventions, maternal and newborn outcomes, and stillbirths. Implementation research is needed to design registers to include necessary data elements and to optimise register filling, local use, and data flow, including linkage to electronic platforms already used in low- and middle-income countries (LMICs) [33]. Capturing detailed aspects for quality of care is not likely to be feasible in routine registers, and it requires specific linked datasets (e.g. on neonatal care wards). Timing components such as early initiation of breastfeeding, uterotonic administration, and resuscitation are challenging to record and may need special studies. More research is required on ways to capture and improve the delays in service delivery found in this study, since such delays can cause deaths.
The ENAP measurement improvement roadmap published in 2015 was instrumental in bringing together a wide team and undertaking the important yet challenging EN-BIRTH study. The World Health Organization (WHO) and United Nations Children's Fund (UNICEF) with ENAP partners are reviewing these findings, alongside other evidence, to update recommendations on newborn indicators, including on the metadata (i.e. definition, numerators/denominators and recommended data collection platforms). This work is crucial given the launch of new ENAP coverage targets for all countries from 2020 to 2025, including a novel target for small and sick newborn care. In addition, the ENAP measurement improvement roadmap will be updated to set out clear priorities for research in the next 5 years.
As well as being an ambitious research study, the EN-BIRTH team is an example of an equitable partnership, with built-in opportunities for multi-directional learning across study sites. At least four linked PhDs are being undertaken by researchers participating in the study. Given commitments to decolonising global health, institutions and funders should support other studies that build capacity of in-country teams for leadership and analytical skills. More data alone will not change outcomes – we need to foster the next generation of leaders and researchers to improve the data, and to use data in the highest burden settings.
EN-BIRTH study shows that a large increase in data on maternal and newborn health could be achieved by strengthening routine health information systems, enabling improved clinical care, and better tracking towards the ENAP coverage targets and ultimately the SDGs. With the right actions in the next few years, we can improve data and most importantly increase coverage, equity, and quality of care to save the lives of every mother and every newborn, everywhere.
Supplementary Information
Additional file 1. Key findings of EN-BIRTH study.
Acknowledgements
Our thanks to Joy E. Lawn, Louise T. Day, Harriet Ruysen, Kimberly Peven and Stefanie Kong for their support to develop this editorial. We appreciate the whole EN-BIRTH team in the three countries, the national advisory groups, and the global Expert Advisory Group linked to Every Newborn and Ending Preventable Maternal Mortality. We commend CIFF for funding this study, including linked PhDs. Lastly, and most importantly, we thank the women and their families who were part of EN-BIRTH study, the health workers, and the data collectors.
We acknowledge the following groups:
National Advisory Groups:
Bangladesh: Mohammod Shahidullah, Khaleda Islam, Md Jahurul Islam.
Nepal: Naresh P KC, Parashu Ram Shrestha.
Tanzania: Muhammad Bakari Kambi, Georgina Msemo, Asia Hussein, Talhiya Yahya, Claud Kumalija, Eliudi Eliakimu, Mary Azayo, Mary Drake, Honest Kimaro.
EN-BIRTH validation collaborative group:
Bangladesh: Md. Ayub Ali, Bilkish Biswas, Rajib Haider, Md. Abu Hasanuzzaman, Md. Amir Hossain, Ishrat Jahan, Rowshan Hosne Jahan, Jasmin Khan, M A Mannan, Tapas Mazumder, Md. Hafizur Rahman, Md. Ziaul Haque Shaikh, Aysha Siddika, Taslima Akter Sumi, Md. Taqbir Us Samad Talha.
Tanzania: Evelyne Assenga, Claudia Hanson, Edward Kija, Rodrick Kisenge, Karim Manji, Fatuma Manzi, Namala Mkopi, Mwifadhi Mrisho, Andrea Pembe.
Nepal: Jagat Jeevan Ghimire, Rejina Gurung, Elisha Joshi, Avinash K Sunny, Naresh P. KC, Nisha Rana, Shree Krishna Shrestha, Dela Singh, Parashu Ram Shrestha, Nishant Thakur.
LSHTM: Hannah Blencowe, Sarah G Moxon.
EN-BIRTH Expert Advisory Group: Agbessi Amouzou, Tariq Azim, Debra Jackson, Theopista John Kabuteni, Matthews Mathai, Jean-Pierre Monet, Allisyn C. Moran, Pavani K. Ram, Barbara Rawlins, Jennifer Requejo, Johan Ivar Sæbø, Florina Serbanescu, Lara Vaz.
About this supplement
This article has been published as part of BMC Pregnancy and Childbirth Volume 21 Supplement 1, 2021: Every Newborn BIRTH multi-country validation study: informing measurement of coverage and quality of maternal and newborn care. The full contents of the supplement are available online at https://bmcpregnancychildbirth.biomedcentral.com/articles/supplements/volume-21-supplement-1.
Abbreviations
- CIFF
Children’s Investment Fund Foundation
- ENAP
Every Newborn Action Plan
- EN-BIRTH
Every Newborn-Birth Indicators Research Tracking in Hospitals study
- icddr,b
International Centre for Diarrhoeal Disease Research, Bangladesh
- IHI
Ifakara Health Institute
- LMIC
low- and middle-income countries
- KMC
kangaroo mother care
- LSHTM
London School of Hygiene & Tropical Medicine
- MUHAS
Muhimbili University of Health and Allied Sciences
- SDG
Sustainable Development Goal
- UNICEF
United Nations International Children’s Emergency Fund
- WHO
World Health Organization
Authors’ contributions
The EN-BIRTH study overall was conceived by JEL, who acquired the funding and led the overall design. Each of the three-country research teams contributed to the development of all data collection tools, review processes, data collection and quality assurance. The icddr,b team (notably SEA, AER, TT, TH, QSR, SBZ, SA) led the development of the software application, data dashboards and database development with VG and the LSHTM team. QSR was the main lead for data management working closely with LTD. IHI and MUHAS team coordinated work on barriers and enablers for data collection and used, working closely with LTD. This manuscript was drafted by AM and JR. The author’s views are their own, and not necessarily from any of the institutions they represent, including WHO and UNICEF. The authors read and approved the final manuscript.
EN-BIRTH Study Group:
Bangladesh: Ahmed Ehsanur Rahman, Tazeen Tahsina, Sojib Bin Zaman, Shafiqul Ameen, Abu Bakkar Siddique, Aniqa Tasnim Hossain, Tapas Mazumder, Jasmin Khan, Taqbir Us Samad Talha, Rajib Haider, Md. Hafizur Rahman, Anisuddin Ahmed, Tanvir Hossain, Qazi Sadeq-ur Rahman, Shams El Arifeen.
Nepal: Omkar Basnet, Avinash K Sunny, Nishant Thakur, Rejina Gurung, Anjani Kumar Jha, Bijay Jha, Ram Chandra Bastola, Rajendra Paudel, Asmita Paudel, Ashish KC.
Tanzania: Nahya Salim, Donat Shamba, Josephine Shabani, Kizito Shirima, Menna Narcis Tarimo, Godfrey Mbaruku (deceased), Honorati Masanja.
LSHTM: Louise T Day, Harriet Ruysen, Kimberly Peven, Vladimir Sergeevich Gordeev, Georgia R Gore-Langton, Dorothy Boggs, Stefanie Kong, Angela Baschieri, Simon Cousens, Joy E Lawn.
Funding
The Children’s Investment Fund Foundation (CIFF) is the main funder of the EN-BIRTH study, which is administered via The London School of Hygiene & Tropical Medicine. The Swedish Research Council specifically funded the Nepal site through Lifeline Nepal and Golden Community. We acknowledge the core funders for all the partner institutions. Publication of this manuscript has been funded by CIFF. CIFF attended the study design workshop but had no role in data collection, analysis, data interpretation, report writing or decision to submit for publication. The corresponding author had full access to study data and final responsibility for publication submission decision.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available on LSHTM Data Compass repository, https://datacompass.lshtm.ac.uk/955/.
Ethics approval and consent to participate
This study was granted ethical approval by institutional review boards in all operating counties in addition to the London School of Hygiene & Tropical Medicine. Voluntary informed written consent was obtained from all women (primary caregivers of newborns treated for infection), who were assured of anonymity and confidentiality. All women were provided with a description of the study procedures in their preferred language before abstraction of data from hospital inpatient case notes and offered the right to refuse or withdraw consent at any time during the data collection process. Voluntary informed written consent was obtained from the respondents (health service providers and data collectors) for the qualitative interviews who were assured of anonymity and confidentiality. EN-BIRTH is study number 4833, registered at https://www.researchregistry.com.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.United Nations Inter-agency Group for Child Mortality Estimation (UN IGME) Levels & Trends in Child Mortality 2020, Estimates developed by the United Nations Inter-agency Group for Child Mortality Estimation. New York: United Nations Children’s Fund; 2020. [Google Scholar]
- 2.United Nations Inter-agency Group for Child Mortality Estimation (UN IGME) A Neglected Tragedy: The global burden of stillbirths. New York: United Nations Children’s Fund; 2020. [Google Scholar]
- 3.Trends in maternal mortality 2000 to 2017: estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. World Health Organization, Geneva, 2019. [https://www.who.int/reproductivehealth/publications/maternal-mortality-2000-2017/en/]. Accessed 05 Nov 2020.
- 4.Lawn JE, Blencowe H, Oza S, You D, Lee AC, Waiswa P, Lalli M, Bhutta Z, Barros AJ, Christian P, et al. Every Newborn: progress, priorities, and potential beyond survival. Lancet. 2014;384(9938):189–205. doi: 10.1016/S0140-6736(14)60496-7. [DOI] [PubMed] [Google Scholar]
- 5.UNICEF, WHO . Every Newborn: an action plan to end preventable deaths. Geneva: WHO; 2014. [Google Scholar]
- 6.The Lancet Every Newborn Series. Lancet. 2014. https://www.thelancet.com/series/everynewborn. Accessed 05 Nov 2020.
- 7.United Nations: Sustainable development goals. [http://www.un.org/sustainabledevelopment/sustainable-development-goals/]. Accessed 05 Nov 2020.
- 8.Ashish KC, Gurung R, Kinney MV, Sunny AK, Moinuddin M, Basnet O, Paudel P, Bhattarai P, Subedi K, Shrestha MP, et al. Effect of the COVID-19 pandemic response on intrapartum care, stillbirth, and neonatal mortality outcomes in Nepal: a prospective observational study. Lancet Glob Health. 2020;8(10):e1273–e1281. doi: 10.1016/S2214-109X(20)30345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mason E, McDougall L, Lawn JE, Gupta A, Claeson M, Pillay Y, Presern C, Lukong MB, Mann G, Wijnroks M, et al. From evidence to action to deliver a healthy start for the next generation. Lancet. 2014;384(9941):455–467. doi: 10.1016/S0140-6736(14)60750-9. [DOI] [PubMed] [Google Scholar]
- 10.Moxon SG, Ruysen H, Kerber KJ, Amouzou A, Fournier S, Grove J, Moran AC, Vaz LM, Blencowe H, Conroy N. Count every newborn; a measurement improvement roadmap for coverage data. BMC Pregnancy Childbirth. 2015;15(2):S8. doi: 10.1186/1471-2393-15-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ENAP measurement improvement roadmap. [https://www.healthynewbornnetwork.org/issues/global-initiatives/enap-metrics/]. Accessed 05 Nov 2020.
- 12.Day LT, Ruysen H, Gordeev VS, Gore-Langton GR, Boggs D, Cousens S, Moxon SG, Blencowe H, Baschieri A, Rahman AE, et al. "Every Newborn-BIRTH" protocol: observational study validating indicators for coverage and quality of maternal and newborn health care in Bangladesh, Nepal and Tanzania. J Glob Health. 2019;9(1):010902. [DOI] [PMC free article] [PubMed]
- 13.Healthy Newborn Network: Database: Global and National Newborn Health Data and Indicators. 2020. [https://www.healthynewbornnetwork.org/resource/database-global-and-national-newborn-health-data-and-indicators]. Accessed 3 Dec 2020.
- 14.Day LT, Rahman QS, Rahman AE, Salim N, KC A, Ruysen H, Tahsina T, Masanja H, Basnet O, Gore-Langton GR et al. Assessment of the validity of the measurement of newborn and maternal health-care coverage in hospitals (EN-BIRTH): an observational study. Lancet Glob Health 2020. 10.1016/S2214-109X(20)30504-0. [DOI] [PubMed]
- 15.Ruysen H, Rahman AE, Gordeev VS, Hossain T, Basnet O, Shirima K, Rahman QS, Zaman SB, Rana N, Salim N et al: Electronic data collection for multi-country, hospital-based clinical observation of maternal and newborn care: experiences from the EN-BIRTH study BMC Pregnancy Childbirth. 2021. 10.1186/s12884-020-03426-5. [DOI] [PMC free article] [PubMed]
- 16.Ameen S, Siddique AB, Peven K, Rahman QS, Day LT, Shabani J, KC A, Boggs D, Shamba D, Tahsina T et al: Survey of women’s report for 33 maternal & newborn indicators: EN-BIRTH multi-country validation study BMC Pregnancy Childbirth. 2021. 10.1186/s12884-020-03425-6. [DOI] [PMC free article] [PubMed]
- 17.Shamba D, Day LT, Zaman SB, Sunny AK, Tarimo MN, Peven K, Khan J, Thakur N, Talha MTUS, KC A et al: Barriers and enablers to routine register data collection for newborns and mothers: EN-BIRTH multi-country study. BMC Pregnancy Childbirth. 2021. 10.1186/s12884-020-03517-3. [DOI] [PMC free article] [PubMed]
- 18.Ruysen H, Shabani J, Hanson C, Day LT, Pembe AB, Peven K, Rahman QS-u, Thakur N, Sharma K, Tahsina T et al: Uterotonics for prevention of postpartum haemorrhage: EN-BIRTH multi-country validation study. BMC Pregnancy Childbirth. 2021. 10.1186/s12884-020-03420-x. [DOI] [PMC free article] [PubMed]
- 19.Tahsina T, Hossain AT, Ruysen H, Rahman AE, Day LT, Peven K, Rahman QS-u, Khan J, Shabani J, KC A et al: Immediate newborn care and breastfeeding: EN-BIRTH multi-country validation study BMC Pregnancy Childbirth. 2021. 10.1186/s12884-020-03421-w. [DOI] [PMC free article] [PubMed]
- 20.Zaman SB, Siddique AB, Ruysen H, KC A, Peven K, Ameen S, Thakur N, Rahman QS-u, Salim N, Gurung R et al: Chlorhexidine for facility-based umbilical cord care: EN-BIRTH multi-country validation study BMC Pregnancy Childbirth. 2021. 10.1186/s12884-020-03338-4. [DOI] [PMC free article] [PubMed]
- 21.KC A, Peven K, Ameen S, Msemo G, Basnet O, Ruysen H, Zaman SB, Mkony M, Sunny AK, Rahman QS-u et al: Neonatal resuscitation: EN-BIRTH multi-country validation study BMC Pregnancy Childbirth. 2021. 10.1186/s12884-020-03422-9. [DOI] [PMC free article] [PubMed]
- 22.Salim N, Shabani J, Peven K, Rahman QS-u, KC A, Shamba D, Ruysen H, Rahman AE, KC N, Mkopi N et al: Kangaroo mother care: EN-BIRTH multi-country validation study BMC Pregnancy Childbirth. 2021. 10.1186/s12884-020-03423-8. [DOI] [PMC free article] [PubMed]
- 23.Rahman AE, Hossain AT, Ameen S, Salim N, KC A, Day LT, Kija E, Peven K, Tahsina T, Zaman SB et al: Antibiotic use for inpatient newborn care with suspected infection: EN-BIRTH multi-country validation study. BMC Pregnancy Childbirth 2021. 10.1186/s12884-020-03424-7. [DOI] [PMC free article] [PubMed]
- 24.Kong S, Day LT, Zaman SB, Peven K, Salim N, Sunny AK, Shamba D, Rahman QS-u, KC A, Ruysen H et al: Birthweight: EN-BIRTH multi-country validation study. BMC Pregnancy Childbirth. 2021. 10.1186/s12884-020-03355-3. [DOI] [PMC free article] [PubMed]
- 25.Peven K, Day LT, Ruysen H, Tahsina T, KC A, Shabani J, Kong S, Ameen S, Basnet O, Haider R et al: Stillbirths including intrapartum timing: EN-BIRTH multi-country validation study. BMC Pregnancy Childbirth. 2021. 10.1186/s12884-020-03238-7. [DOI] [PMC free article] [PubMed]
- 26.Gurung R, Day LT, Sunny AK, Penn-Kekana L, Målqvist M, Ghimire B, Bastola RC, Singh D, Basnet O, Sharma S et al: Respectful maternal and newborn care: measurement in one EN-BIRTH study hospital in Nepal. BMC Pregnancy Childbirth. 2021. 10.1186/s12884-020-03516-4. [DOI] [PMC free article] [PubMed]
- 27.Gladstone ME, Salim N, Ogillo K, Shamba D, Gore-Langton GR, Day LT, Blencowe H, Lawn JE, EN-BIRTH Study Group: Birthweight measurement processes and perceived value: qualitative research in one EN-BIRTH study hospital in Tanzania BMC Pregnancy Childbirth. 2021. 10.1186/s12884-020-03356-2. [DOI] [PMC free article] [PubMed]
- 28.Reed S, Shabani J, Boggs D, Salim N, Ng'unga S, Day LT, Peven K, Kong S, Ruysen H, Jackson D et al: Counting on birth registration: mixed-methods research in two EN-BIRTH study hospitals in Tanzania. BMC Pregnancy Childbirth. 2021. 10.1186/s12884-020-03357-1. [DOI] [PMC free article] [PubMed]
- 29.Campbell H, El Arifeen S, Hazir T, O'Kelly J, Bryce J, Rudan I, Qazi SA. Measuring coverage in MNCH: challenges in monitoring the proportion of young children with pneumonia who receive antibiotic treatment. PLoS Med. 2013;10(5):e1001421. doi: 10.1371/journal.pmed.1001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blanc AK, Diaz C, McCarthy KJ, Berdichevsky K. Measuring progress in maternal and newborn health care in Mexico: validating indicators of health system contact and quality of care. BMC Pregnancy Childbirth. 2016;16(1):255. doi: 10.1186/s12884-016-1047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blanc AK, Warren C, McCarthy KJ, Kimani J, Ndwiga C, RamaRao S. Assessing the validity of indicators of the quality of maternal and newborn health care in Kenya. J Glob Health. 2016;6(1):010405. doi: 10.7189/jogh.06.010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sando D, Abuya T, Asefa A, Banks KP, Freedman LP, Kujawski S, Markovitz A, Ndwiga C, Ramsey K, Ratcliffe H, et al. Methods used in prevalence studies of disrespect and abuse during facility based childbirth: lessons learned. Reprod Health. 2017;14(1):127. doi: 10.1186/s12978-017-0389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Health Information Systems Program: District Health Information Software 2 (DHIS 2). [https://www.dhis2.org/]. Accessed 05 Nov 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Key findings of EN-BIRTH study.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available on LSHTM Data Compass repository, https://datacompass.lshtm.ac.uk/955/.