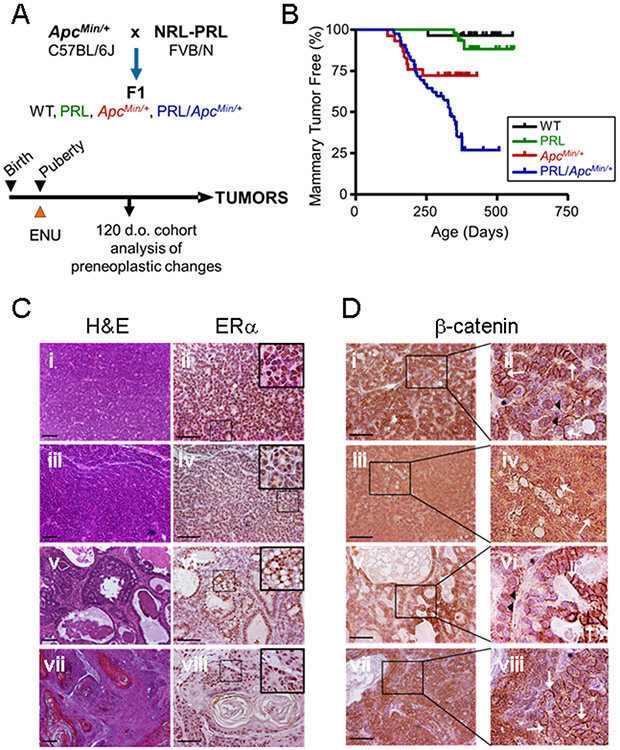
Fig. 1. Prolactin potentiates tumorigenesis in the ApcMin/+ background.
(A) Experimental Design. Heterozygous NRL-PRL FVB/N females were crossed with ApcMin/+ C57BL/6Jmales, generating the four genotypes in the F1 background. Pubertal mice were injected with ENU as described in the Methods. A cohort of age-matched animals was examined at 120 days of age, and the remainder allowed to age until end stage. (B) The PRL transgene increased mammary tumor incidence in the ApcMin/+ background (ApcMin/+ vs. NRL-PRL/ApcMin/+, p=0.008, logrank test), but not tumor latency (Kaplan-Meier analysis, followed by Mantel-Cox test, ns). N= WT, 28; NRL-PRL, 28; ApcMin/+, 30; NRL-PRL/ApcMin/+, 40. (C) Tumors that developed in ApcMin/+ and NRL-PRL/ApcMin/+ females displayed a similar range of histotypes, and abundant ERα expression. Tumors in NRL-PRL/ApcMin/+ shown; ApcMin/+ tumors shown in Suppl. Fig. S1A, and NRL-PRL alone in Suppl. Fig. S1C. Left, hematoxylin and eosin (H&E) stained representative histotypes (i, ii, glandular; iii, iv, microacinar; v, vi, papillary; vii, viii, adenosquamous). Right, ERα expression by immunohistochemistry; insets as shown. (D) Tumors that developed in ApcMin/+ and NRL-PRL/ApcMin/+ females exhibited similarly heterogeneous cellular localization of β-catenin; insets as shown. Tumors in NRL-PRL/ApcMin/+ shown; ApcMin/+ tumors in Suppl. Fig. S1B, and NRL-PRL alone in Suppl. Fig. S1D. (i, ii, glandular; iii, iv, microacinar; v, vi, papillary; vii, viii, adenosquamous). White arrows indicate β-catenin at cell junctions, black arrowheads point to nuclear β-catenin. Black asterisks denote cells with little detectable β-catenin, at background. Scale bars, 100 μm. Original magnifications, H&E, x 100; ERα, β-catenin x 200.