Fig. 3. PRL increases the size, but not the number of epithelial hyperplasias in the ApcMin/+ genetic background.
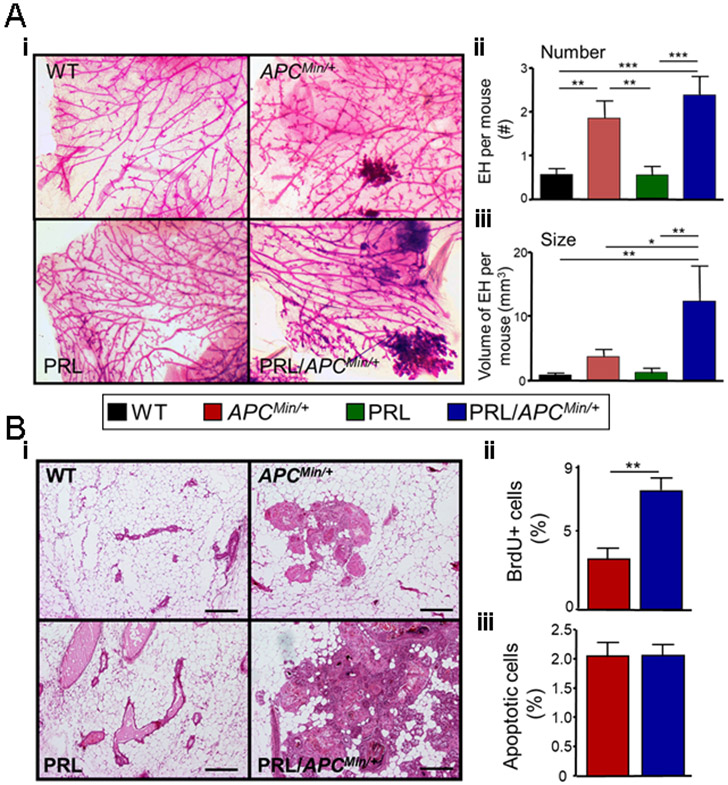
Pubertal females of the four genotypes were treated with ENU, and at 120 days of age, mammary glands were examined (Fig. 1A). (A) Mammary glands from ApcMin/+ and NRL-PRL/ApcMin/+ females exhibited large hyperplastic lesions, compared to WT or NRL-PRL females. (i) Representative micrographs of whole mounted mammary glands stained with carmine alum (genotypes as shown). (ii) ApcMin/+ and NRL-PRL/ApcMin/+ glands contained similar numbers of large lesions. [Number of large epithelial hyperplasias (EH) in the left chain of mammary glands 2-5 of individual females]. (iii) Hyperplasias in NRL-PRL/ApcMin/+ glands were larger than those in ApcMin/+ glands. (Total volume of large EH per mouse, see Methods). (ii, iii) Mean ± S.E.M., N=16-20 mice. Statistical differences among the different genotypes was determined by the Kruskal-Wallis test followed by the Dunn’s Multiple Comparison post test. (*, p<0.05; **, p<0.01; ***, p<0.005). (B) Hyperplastic lesions in NRL-PRL/ApcMin/+ glands proliferated more rapidly than those in ApcMin/+ females. (i) Hematoxylin/ eosin stained glands. Scale bars, 500 μm. Rates of proliferation (ii) and apoptosis (iii) in epithelial cells in large hyperplasias in NRL-PRL/ApcMin/+ and ApcMin/+ glands were determined as described in the Methods. Mean ± S.E.M.; N=6 mice. (**, p<0.01, unpaired Student’s t test.). Rates of apoptosis were not significantly different.
