Keywords: amyotrophic lateral sclerosis, biomarker, creatine kinase, diagnosis, epidemiological study, prognosis, serology, survival
Abstract
Creatine kinase is a muscle enzyme that has been reported at various levels in different studies involving patients with amyotrophic lateral sclerosis. In the present retrospective case-control study, we included 582 patients with amyotrophic lateral sclerosis and 582 age- and sex-matched healthy controls. All amyotrophic lateral sclerosis participants received treatment in the Department of Neurology, West China Hospital, China, between May 2008 and December 2018. Serum creatine kinase levels in patients with amyotrophic lateral sclerosis were significantly higher than those in healthy controls. Subgroup analysis revealed that serum creatine kinase levels in men were higher than those in women in both amyotrophic lateral sclerosis patients and healthy controls. Compared with patients with bulbar-onset amyotrophic lateral sclerosis, patients with limb-onset amyotrophic lateral sclerosis had higher creatine kinase levels. Spearman’s correlation analysis revealed that serum creatine kinase levels were not correlated with body mass index, Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised score, or progression rate. After adjusting for prognostic covariates, higher log creatine kinase values were correlated with higher overall survival in the amyotrophic lateral sclerosis patients. We also investigated the longitudinal changes in serum creatine kinase levels in 81 amyotrophic lateral sclerosis patients; serum creatine kinase levels were decreased at the second blood test, which was sampled at least 6 months after the first blood test. Together, our results suggest that serum creatine kinase levels can be used as an independent factor for predicting the prognosis of amyotrophic lateral sclerosis patients. This study received ethical approval from the Ethics Committee of West China Hospital, China (approval No. 2015(236)) on December 23, 2015.
Chinese Library Classification No. R446; R741; R730.7
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease that is characterized by the selective loss of motor neurons, resulting in muscle atrophy, weakness, and progressive paralysis (Sun et al., 2012; Turner et al., 2013). Differential diagnosis is important because other disorders can mimic ALS, but have different prognoses and treatments (Fogarty, 2019; Riancho et al., 2019). Creatine kinase (CK), a muscle enzyme, is used to differentiate between neurogenic and myopathic disorders. High serum CK levels are related to myopathic disorders, including polymyositis and muscular dystrophy (Munsat et al., 1973). Therefore, measuring serum CK levels during the diagnostic workup may be helpful for differentiating ALS from primary muscular disorders. However, previous investigations of serum CK levels have led to inconsistent results. Elevated serum CK has been reported in 23–100% of patients with ALS (Panitch and Franklin, 1972; Amrit and Anderson, 1974; Edmonds and Ziegler, 1975; Harrington et al., 1983; Sinaki and Mulder, 1986; Felice and North, 1998; Iłzecka and Stelmasiak, 2003; Lima et al., 2003; Süssmuth et al., 2003; Chahin and Sorenson, 2009). Additionally, some studies have reported significantly higher CK levels in men than in women (Amrit and Anderson, 1974; Felice and North, 1998), whereas another study found that the number of ALS patients with increased CK levels was not different between sexes (Lima et al., 2003). Compared with patients with bulbar-onset ALS, CK values are reported to be higher in ALS patients with limb onset (Amrit and Anderson, 1974; Felice and North, 1998). Moreover, some studies have reported that there is no association between CK values and symptom duration, weakness severity, or age of onset, and that serum CK levels are not related to survival (Amrit and Anderson, 1974; Sinaki and Mulder, 1986; Felice and North, 1998; Lima et al., 2003). However, a recent study found that lower serum CK levels are associated with longer overall survival, while another study reported that higher log CK is related to better prognosis and survival in ALS patients (Gibson et al., 2015; Rafiq et al., 2016). Furthermore, a longitudinal study revealed that CK values recorded during the later course of the disease are not significantly different from the values recorded earlier in the disease (Felice and North, 1998), while another study observed an increase in serum CK over the first 18 months of the disease (Süssmuth et al., 2003).
The inconsistencies in these results suggest that more in-depth research into serum CK levels in ALS patients is needed. Thus, the present study aimed to evaluate the differences in serum CK levels between patients with ALS and healthy controls (HCs) from a Chinese population. We also sought to investigate the associations between this muscle-related enzyme and other serological and clinical factors; examine CK levels in ALS patients with different ages, sexes, and onset locations; explore the correlations between CK and functional disability and survival; and study any changes in concentration as the disease progresses.
Participants and Methods
Participants
This retrospective matched case-control study and longitudinal study was performed from May 2008 to December 2018 in the Department of Neurology, Sichuan University West China Hospital, China. A total of 582 ALS patients were included. Patients with clinical and electrophysiological evidence of combined upper and lower motor neuron involvement were included, and all included patients fulfilled the El Escorial revised criteria for definite or probable ALS (Brooks et al., 2000). The exclusion criteria were as follows: patients met the criteria for ALS-frontotemporal dementia (Faber, 1999) or had a history of other neurological conditions that might affect assessment (brain injury, stroke, alcohol-/substance-related disorders, or experiencing depression or major psychiatric disease). Patients with impaired renal function or mutations in C9ORF72 (GGGGCC repeat expansion), TARDBP, FUS, or SOD1 were also excluded (Chen et al., 2019). Additionally, 582 age- and sex-matched HCs were recruited from the Medical Examination Center of West China Hospital. All participants gave their written informed consent (Additional file 1 (1.6MB, pdf) ). The Ethical Committee of West China Hospital approved this study (approval No. 2015(236) on December 23, 2015 (Additional file 2 (267.2KB, pdf) ). Figure 1 shows a flow chart of the study design.
Figure 1.
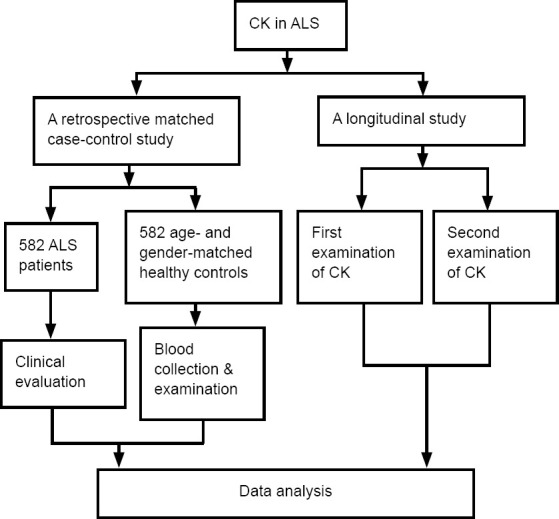
Flow chart of the study design.
The time interval between the first and second CK tests was > 6 months. ALS: Amyotrophic lateral sclerosis; CK: creatine kinase.
Blood sample collection
Peripheral blood samples from the cubital vein were acquired from each ALS patient and HC participant. Samples were taken by venipuncture, performed between 9:00 and 11:00 a.m., after fasting from midnight. Hematological variables were examined using an automatic biochemistry analyzer (Roche Cobas 8000; Rui An International Co. Ltd., Taipei, China). The levels of creatinine, uric acid (UA), total protein, albumin, triglycerides, total cholesterol, high-density lipoprotein, and low-density lipoprotein (LDL) in all participants were determined in our previous study (Chen et al., 2019).
Clinical evaluations
Demographic features were collected from each ALS patient and HC participant, including age, sex, body mass index (BMI), date of registration, date of birth, and personal history. Disease-related variables, such as age of onset, onset form, baseline ALS Functional Rating Scale-Revised (ALSFRS-R) score (Kollewe et al., 2008), disease duration, and treatment were also recorded. The survival time was defined as the interval time between date of onset and date of death or tracheotomy, which was given to 5 ALS patients. Disease progression rate was evaluated using alterations in the ALSFRS-R score each month (Kollewe et al., 2008). If a patient had an age of onset < 45 years, they were considered to have “early-onset” disease. Patients were classified by onset form into limb- and bulbar-onset subgroups. Patients were followed up by our neurologists at 3-month intervals, and the data collected during follow-up included current clinical manifestations, ALSFRS-R score, body weight, medications, and clinical interventions.
Statistical analysis
Comparisons of continuous variables were performed using the Student’s t-test. If data were not parametrically distributed, non-parametric tests were applied. The chi-squared test was used to compare categorical variables. Spearman’s correlation was used to examine the associations between serum CK levels and clinical factors, including ALSFRS-R, progression rate, BMI, and disease duration (Wei et al., 2018). We also analyzed the associations between CK and other serological variables, including UA, creatinine, albumin, total protein, and lipid profile (triglycerides, LDL, high-density lipoprotein, and total cholesterol). The effect of baseline log CK on survival was assessed using the Cox regression analysis, and the adjusted confounding factors included age of onset, onset form, BMI, ALSFRS-R score, and disease duration. The paired t-test was applied to compare the differences between serum CK levels measured at different times; the interval between the first and second examinations was > 6 months. All data were statistically analyzed using SPSS software (version 17.0, SPSS, Chicago, IL, USA), and P < 0.05 was considered to be statistically significant.
Results
Serum CK levels in ALS patients and HCs
The ALS patients were 53.23 ± 11.69 years old at the time of examination. Additionally, 56.87% of patients were men, and 22.85% of patients had bulbar onset. Compared with the HCs, ALS patients had significantly higher serum CK levels (P < 0.0001; Table 1).
Table 1.
Clinical features and serum CK levels in ALS patients and healthy controls
| ALS (n = 582) | Controls (n = 582) | P-value | |
|---|---|---|---|
| Age at examination (yr) | 53.23±11.69 | 53.81±14.47 | 0.454 |
| Male/female | 331/251 | 310/272 | 0.239 |
| Body mass index (kg/m2) | 22.17±2.96 | 23.69±4.53 | 0.783 |
| Bulbar onset/limb onset | 133/449 | – | – |
| ALS Functional Rating Scale-Revised score | 38.14±6.58 | – | – |
| Progression rate | 0.83±0.69 | – | – |
| Disease duration (mon) | 16.36±14.55 | – | – |
| CK (U/L) | 169.7±137.7 | 78.71±49.85 | < 0.0001 |
Data are expressed as the mean ± SD, except sex and bulbar onset/limb onset, which are expressed as numbers. All data were analyzed using the Student's t-test. ALS: Amyotrophic lateral sclerosis; CK: creatine kinase.
Subgroup analyses of CK levels by sex, age of onset, and onset form
In the subgroup analyses, serum CK levels were significantly higher in men than in women in both ALS patients and HCs (P < 0.0001). Compared with the patients with bulbar-onset ALS, patients with limb-onset ALS had higher CK levels (P = 0.0001). There were no significant differences in serum CK levels between early- and late-onset patients (P = 0.7210; Figure 2).
Figure 2.
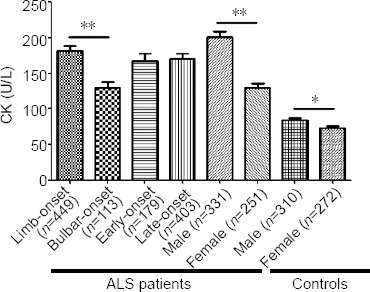
Subgroup analysis of serum CK levels in ALS patients and controls.
Data are expressed as the mean ± SD and analyzed using the Student’s t-test. *P = 0.0064, **P < 0.0001. ALS: Amyotrophic lateral sclerosis; CK: creatine kinase.
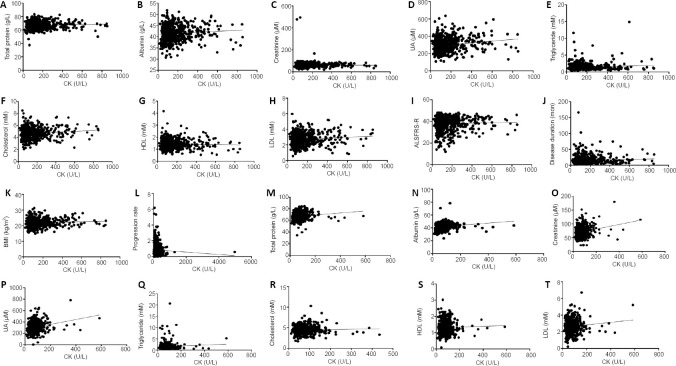
The correlation analysis between CK levels and other serological variables demonstrated that serum creatinine, UA, total protein, albumin, LDL, and cholesterol levels were significantly positively correlated with serum CK levels in both ALS patients and HCs (Figure 3). Additionally, in ALS patients, CK levels were independently correlated with disease duration, but were not correlated with ALSFRS-R score, BMI, or progression rate (Figure 3).
Figure 3.
Correlation analysis between CK and other serological variables in ALS patients and controls.
(A–L) Correlation analysis between CK and other serological variables in ALS patients. CK in the ALS patients was positively correlated with total protein (r = 0.1028, P = 0.0132; A), albumin (r = 0.1339, P = 0.0012; B), creatinine (r = 0.0012, P = 0.0287; C), UA (r = 0.1916, P < 0.0001; D), cholesterol (r = 0.1133, P = 0.0063; F), LDL (r = 0.09074, P = 0.0287; H), and disease duration (r = 0.1200, P = 0.0037; J). CK in the ALS patients was not correlated with triglycerides (r = 0.03849, P = 0.3544; E), HDL (r = –0.01237, P = 0.7660; G), ALSFRS-R (r = –0.01015, P = 0.8070; I), BMI (r = 0.04862, P = 0.2415; K), or progression rate (r = –0.06878, P = 0.0974; L). (M–T) Correlation analysis between CK and other serological variables in healthy controls. CK in the healthy controls was positively correlated with total protein (r = 0.1361, P = 0.0011; M), albumin (r = 0.1846, P < 0.0001; N), creatinine (r = 0.2207, P < 0.0001; O), UA (r = 0.2363, P < 0.0001; P), cholesterol (r = 0.1162, P = 0.0055; R), and LDL (r = 0.1003, P = 0.0165; T). CK in the healthy controls was not correlated with triglycerides (r = 0.07056, P = 0.0921; Q) or HDL (r = 0.03044, P = 0.4679; S). All data were analyzed using Spearman’s correlation. ALS: Amyotrophic lateral sclerosis; ALSFRS-R: ALS Functional Rating Scale-Revised; BMI: body mass index; CK: creatine kinase; HDL: high-density lipoprotein; LDL: low-density lipoprotein; UA: uric acid.
The effect of log CK on patient prognosis was then examined. After adjusting for the prognostic covariates, we revealed that a one-unit increase in the log-transformed serum CK value resulted in an ALS patient being 0.623 times more likely to die (95% confidence interval: 0.442–0.879) at any given timepoint. Furthermore, the results of the Cox regression analysis demonstrated that the log CK value was significantly related to overall survival (Table 2).
Table 2.
Results from the Cox proportional hazards survival analysis in ALS patients
| HR (95% CI) | P-value | ||
|---|---|---|---|
| Model I | Log CK (unadjusted) | 0.651 (0.460–0.922) | 0.015 |
| Model II | Log CK (adjusted)a | 0.623 (0.442–0.879) | 0.007 |
n = 371. aAdjusted for age at disease onset, site of disease onset, BMI, ALSFRS-R score, and disease duration. ALS: Amyotrophic lateral sclerosis; ALSFRS-R: ALS Functional Rating Scale-Revised; BMI: body mass index; CI: confidence interval; CK: creatine kinase; HR: hazard ratio.
Eighty-one ALS patients received two blood tests during their disease progression, and longitudinal changes in their serum CK levels were investigated. The time interval between the first and second blood tests was > 6 months in each patient, and CK levels were significantly decreased in the second examination (138.8 ± 132.0 U/L) compared with the first examination (167.4 ± 155.7 U/L; P = 0.0283).
Discussion
We demonstrated that serum CK levels were higher in ALS patients than in HCs. Furthermore, serum CK levels were related to overall survival in ALS patients. Levels of CK did not correlate with disease severity or progression rate but did correlate with disease duration; moreover, CK levels decreased over time.
In the present study, the range of CK values (17–853 U/L) in the ALS patients was similar to those reported in previous studies (Panitch and Franklin, 1972; Amrit and Anderson, 1974; Edmonds and Ziegler, 1975; Harrington et al., 1983; Sinaki and Mulder, 1986; Felice and North, 1998; Iłzecka and Stelmasiak, 2003; Lima et al., 2003; Chahin and Sorenson, 2009; Lu et al., 2016), in which 23–70% of ALS patients were reported to have elevated serum CK levels, and 29% of patients had levels above the upper limit of the normal range (Harrington et al., 1983; Sinaki and Mulder, 1986; Iłzecka and Stelmasiak, 2003; Rafiq et al., 2016). In the present study, 92% of patients had a serum CK concentration < 400 U/L, indicating that ALS patients normally have mildly or moderately elevated CK levels, unlike patients with muscle diseases. Furthermore, previous studies have reported that CK elevation is significantly higher and more common in patients with spinal and bulbar muscular atrophy compared with ALS patients (Chahin and Sorenson, 2009; Lombardi et al., 2019). Hence, it is suggested that individuals with a CK concentration above 1000 U/L and who are suspected to have ALS should undergo a detailed evaluation for differential diagnosis, and alternative explanations should be considered.
The pathogenesis of CK elevation in ALS patients remains unclear. Proposed explanations include the following: (1) the degeneration of innervating motor neurons leads to active muscle denervation, which might contribute to the elevation of CK in ALS (Tai et al., 2018); (2) elevated endogenous ATP activity in the mitochondria, caused by muscle energy metabolism disturbance, leads to an upregulation of CK expression (Rafiq et al., 2016); and (3) myopathic changes, which have been historically demonstrated (Achari and Anderson, 1974; Chahin and Sorenson, 2009).
Previous studies have reported significant differences in CK values between male and female ALS patients (Felice and North, 1998; Rafiq et al., 2016; Tai et al., 2017). For the first time, we examined the serum CK levels in both ALS patients and HCs, and CK levels were higher in men than in women. However, Gibson et al. (2015) reported that the differences in CK levels between male and female ALS patients were no longer significant after adjusting for fat-free mass. Furthermore, Rafiq et al. (2016) demonstrated a linear correlation between CK levels and lean body mass. Therefore, the previous findings of CK elevations in male ALS patients are not disease-specific, and the higher CK levels in men may be explained by their higher muscle mass.
In the present study, we also revealed that CK levels were significantly higher in patients with limb-onset ALS than in those with bulbar-onset ALS; this finding has also been reported in previous studies (Felice and North, 1998; Iłzecka and Stelmasiak, 2003; Gibson et al., 2015; Rafiq et al., 2016; Tai et al., 2017). It may be that CK elevation reflects the extent of muscle involvement, suggesting that ALS may affect the muscle fibers; in support of this idea, a study has shown one or more myopathic features in muscle biopsies from ALS patients, although these features were considered to be secondary changes caused by denervation (Achari and Anderson, 1974). ALS patients with limb onset have a higher extent of muscle involvement than patients with bulbar onset, which may contribute to the significant differences in CK values between limb-onset and bulbar-onset patients. Furthermore, after a single muscle cramp in healthy individuals, there is an approximately threefold increase in CK values within 6 hours, and about a fivefold increase after 30 hours (Gilchrist, 2003). Cramps can therefore markedly and transiently elevate serum CK levels. Because cramps are frequent in ALS, elevated CK levels may in part be caused by muscle cramps. Indeed, a previous study found an association between cramps and CK levels: for every one-unit elevation in the cramp index, there was a 15.1 U/L increase in serum CK levels (Gibson et al., 2015). Moreover, another study demonstrated that limb-onset ALS patients had more cramps than bulbar-onset patients (Caress et al., 2016).
In the present study, there was a correlation between CK values and disease duration, but this association was no longer significant after multivariate regression analysis. The significant correlation of “disease duration” was lost when “site of onset” was added to the model. A possible explanation for this finding is that the symptoms of ALS are usually focal at onset, but with the progression of the disease, the signs and manifestations of ALS usually develop in an anatomically contiguous way (Rafiq et al., 2016). An elevated CK level may thus represent an ongoing pathological process in a focal region, but this level is likely to fall with widespread denervation. Additionally, one study showed that the number of muscle cramps tended to be lower after the first year of ALS (Caress et al., 2016).
Some previous studies have reported that serum CK levels are associated with serum creatinine levels in ALS patients (Rafiq et al., 2016; Tai et al., 2017). Additionally, Mitsumoto and Saito (2018) revealed a positive relationship between CK and UA levels in limb-onset ALS patients. The current study is the first to examine the correlations between CK levels and other serological variables in both ALS patients and HCs. We revealed that CK levels were correlated with levels of creatinine, UA, total cholesterol, total protein, albumin, and LDL in both ALS patients and HCs. Although these correlations between CK and other serological variables are not disease-specific, we believe that they are meaningful, because all of these serological factors have been previously reported to be associated with prognosis in ALS. A meta-analysis demonstrated that creatinine may be a promising prognosis biomarker for ALS (Lanznaster et al., 2019); furthermore, a meta-analysis that we conducted also indicated a positive association between serum creatinine levels and overall survival among ALS patients. Our previous study reported that levels of serum UA, total protein, and albumin are all associated with the ALS staging system (Chen et al., 2019). That is, UA, total protein, and albumin levels decline with the progression of ALS from the early to advanced stages. Another study also reported that serum albumin and creatinine are independent markers of outcome in ALS (Chiò et al., 2014). Furthermore, a state of lipid dysregulation is now widely recognized in ALS (Dupuis et al., 2011). Therefore, the close relationships between CK and other serological factors indicate that CK is useful for the survival prognosis of ALS patients.
Previous studies have examined the effects of CK on prognosis in ALS. Two studies reported no association between CK and overall survival (Sinaki and Mulder, 1986; Felice and North, 1998), while one study demonstrated that a lower baseline CK was associated with better survival (Gibson et al., 2015), and two more recent studies showed that patients with higher CK levels at diagnosis had better prognosis (Rafiq et al., 2016; Tai et al., 2017). The inconsistencies among these previous studies may be explained as follows: (1) the sample sizes in some of these studies were relatively small; (2) different laboratory detections and selections of cutoff values may have affected the findings; and (3) the inclusion of ALS patients with different disease durations may have given rise to bias (Gibson et al., 2015). In the present study, we included 582 ALS patients and adjusted for other prognostic factors, and found that higher serum CK levels were associated with better survival. Interestingly, bulbar muscular atrophy patients have higher CK levels than ALS patients, and bulbar muscular atrophy has a much better prognosis than ALS (Chahin and Sorenson, 2009). Thus, our results indicate that high CK levels play a positive role in ALS survival.
The current study had some limitations. First, it was a hospital-based study. Second, the CK longitudinal change data were available from only a small proportion of ALS patients; therefore, the comparison did not represent the entire cohort. Third, the CK values were log transformed, and the effect of primary CK levels on survival should therefore be interpreted cautiously.
In summary, serum CK levels were increased in patients with ALS, and particularly in those with limb-onset forms of the disease. Higher CK levels were associated with a better prognosis, and CK levels decreased as the disease progressed. We hypothesize that elevated CK levels might represent an upregulation of the metabolic pathway and might be an overall protective factor.
Additional files:
Additional file 1 (1.6MB, pdf) : Informed consent form (Chinese).
Additional file 2 (267.2KB, pdf) : Hospital ethics approval (Chinese).
Additional file 3: Open peer review reports 1 (84.3KB, pdf) and 2 (84.5KB, pdf) .
Acknowledgments
We thank all the patients who participated in this study and their families.
Footnotes
P-Reviewers: Gaikwad S, Barnstable CJ; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Gardner D, Yu J, Song LP; T-Editor: Jia Y
Conflicts of interest: The authors declare no competing interests.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81301093 (to XPC). The funder had no roles in the study design, conduction of experiment, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional review board statement: The study was ethically approved by the Ethical Committee of West China Hospital (approval No. 2015(236)) on December 23, 2015.
Declaration of participant consent: The authors certify that they have obtained the consent forms from participants. In the forms, the participants have given their consent for their images and other clinical information to be reported in the journal. The participants understand that their names and initials will not be published and due efforts will be made to conceal their identity.
Reporting statement: This study followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement.
Biostatistics statement: The statistical methods of this study were reviewed by the biostatistician of the West China Hospital of Sichuan University, China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Sagar Gaikwad, University of Texas, USA; Colin J. Barnstable, Penn State Hershey Eye Center, USA.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81301093 (to XPC).
References
- 1.Achari AN, Anderson MS. Myopathic changes in amyotrophic lateral sclerosis. Pathologic analysis of muscle biopsy changes in 111 cases. Neurology. 1974;24:477–481. doi: 10.1212/wnl.24.5.477. [DOI] [PubMed] [Google Scholar]
- 2.Amrit AN, Anderson MS. Serum creatine phosphokinase in amyotrophic lateral sclerosis. Correlation with sex, duration, and skeletal muscle biopsy. Neurology. 1974;24:834–837. doi: 10.1212/wnl.24.9.834. [DOI] [PubMed] [Google Scholar]
- 3.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 4.Caress JB, Ciarlone SL, Sullivan EA, Griffin LP, Cartwright MS. Natural history of muscle cramps in amyotrophic lateral sclerosis. Muscle Nerve. 2016;53:513–517. doi: 10.1002/mus.24892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chahin N, Sorenson EJ. Serum creatine kinase levels in spinobulbar muscular atrophy and amyotrophic lateral sclerosis. Muscle Nerve. 2009;40:126–129. doi: 10.1002/mus.21310. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Wei QQ, Chen Y, Cao B, Ou R, Hou Y, Yuan X, Zhang L, Liu H, Shang H. Clinical disease stage related changes of serological factors in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20:53–60. doi: 10.1080/21678421.2018.1550516. [DOI] [PubMed] [Google Scholar]
- 7.Chiò A, Calvo A, Bovio G, Canosa A, Bertuzzo D, Galmozzi F, Cugnasco P, Clerico M, De Mercanti S, Bersano E, Cammarosano S, Ilardi A, Manera U, Moglia C, Sideri R, Marinou K, Bottacchi E, Pisano F, Cantello R, Mazzini L, et al. Amyotrophic lateral sclerosis outcome measures and the role of albumin and creatinine: a population-based study. JAMA Neurol. 2014;71:1134–1142. doi: 10.1001/jamaneurol.2014.1129. [DOI] [PubMed] [Google Scholar]
- 8.Dupuis L, Pradat PF, Ludolph AC, Loeffler JP. Energy metabolism in amyotrophic lateral sclerosis. Lancet Neurol. 2011;10:75–82. doi: 10.1016/S1474-4422(10)70224-6. [DOI] [PubMed] [Google Scholar]
- 9.Edmonds PJ, Ziegler DK. Diagnostic value of serum creatine phosphokinase in motor neuron disease. South Med J. 1975;68:1388–1390. doi: 10.1097/00007611-197511000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Faber R. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1999;53:1159. doi: 10.1212/wnl.53.5.1158-b. [DOI] [PubMed] [Google Scholar]
- 11.Felice KJ, North WA. Creatine kinase values in amyotrophic lateral sclerosis. J Neurol Sci 160 Suppl. 1998;1:S30–32. doi: 10.1016/s0022-510x(98)00195-6. [DOI] [PubMed] [Google Scholar]
- 12.Fogarty MJ. Amyotrophic lateral sclerosis as a synaptopathy. Neural Regen Res. 2019;14:189–192. doi: 10.4103/1673-5374.244782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibson SB, Kasarskis EJ, Hu N, Pulst SM, Mendiondo MS, Matthews DE, Mitsumoto H, Tandan R, Simmons Z, Kryscio RJ, Bromberg MB. Relationship of creatine kinase to body composition, disease state, and longevity in ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16:473–477. doi: 10.3109/21678421.2015.1062516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilchrist JM. Time course of serum CK immediately before and after a single muscle cramp. Muscle Nerve. 2003;27:766. doi: 10.1002/mus.10383. [DOI] [PubMed] [Google Scholar]
- 15.Harrington TM, Cohen MD, Bartleson JD, Ginsburg WW. Elevation of creatine kinase in amyotrophic lateral sclerosis. Potential confusion with polymyositis. Arthritis Rheum. 1983;26:201–205. doi: 10.1002/art.1780260212. [DOI] [PubMed] [Google Scholar]
- 16.Iłzecka J, Stelmasiak Z. Creatine kinase activity in amyotrophic lateral sclerosis patients. Neurol Sci. 2003;24:286–287. doi: 10.1007/s10072-003-0158-3. [DOI] [PubMed] [Google Scholar]
- 17.Kollewe K, Mauss U, Krampfl K, Petri S, Dengler R, Mohammadi B. ALSFRS-R score and its ratio: a useful predictor for ALS-progression. J Neurol Sci. 2008;275:69–73. doi: 10.1016/j.jns.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Lanznaster D, Bejan-Angoulvant T, Patin F, Andres CR, Vourc’h P, Corcia P, Blasco H. Plasma creatinine and amyotrophic lateral sclerosis prognosis: a systematic review and meta-analysis. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20:199–206. doi: 10.1080/21678421.2019.1572192. [DOI] [PubMed] [Google Scholar]
- 19.Lima AF, Evangelista T, de Carvalho M. Increased creatine kinase and spontaneous activity on electromyography, in amyotrophic lateral sclerosis. Electromyogr Clin Neurophysiol. 2003;43:189–192. [PubMed] [Google Scholar]
- 20.Lombardi V, Querin G, Ziff OJ, Zampedri L, Martinelli I, Heller C, Foiani M, Bertolin C, Lu CH, Malik B, Allen K, Rinaldi C, Zetterberg H, Heslegrave A, Greensmith L, Hanna M, Soraru G, Malaspina A, Fratta P. Muscle and not neuronal biomarkers correlate with severity in spinal and bulbar muscular atrophy. Neurology. 2019;92:e1205–1211. doi: 10.1212/WNL.0000000000007097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu CH, Allen K, Oei F, Leoni E, Kuhle J, Tree T, Fratta P, Sharma N, Sidle K, Howard R, Orrell R, Fish M, Greensmith L, Pearce N, Gallo V, Malaspina A. Systemic inflammatory response and neuromuscular involvement in amyotrophic lateral sclerosis. Neurol Neuroimmunol Neuroinflamm. 2016;3:e244. doi: 10.1212/NXI.0000000000000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitsumoto H, Saito T. A prognostic biomarker in amyotrophic lateral sclerosis. Rinsho Shinkeigaku. 2018;58:729–736. doi: 10.5692/clinicalneurol.cn-001220. [DOI] [PubMed] [Google Scholar]
- 23.Munsat TL, Baloh R, Pearson CM, Fowler W., Jr Serum enzyme alterations in neuromuscular disorders. JAMA. 1973;226:1536–1543. [PubMed] [Google Scholar]
- 24.Panitch HS, Franklin GM. Elevation of serum creatine phosphokinase in amyotrophic lateral sclerosis. Neurology. 1972;22:964–966. doi: 10.1212/wnl.22.9.964. [DOI] [PubMed] [Google Scholar]
- 25.Rafiq MK, Lee E, Bradburn M, McDermott CJ, Shaw PJ. Creatine kinase enzyme level correlates positively with serum creatinine and lean body mass, and is a prognostic factor for survival in amyotrophic lateral sclerosis. Eur J Neurol. 2016;23:1071–1078. doi: 10.1111/ene.12995. [DOI] [PubMed] [Google Scholar]
- 26.Riancho J, Gil-Bea FJ, Santurtun A, López de Munaín A. Amyotrophic lateral sclerosis: a complex syndrome that needs an integrated research approach. Neural Regen Res. 2019;14:193–196. doi: 10.4103/1673-5374.244783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinaki M, Mulder DW. Amyotrophic lateral sclerosis: relationship between serum creatine kinase level and patient survival. Arch Phys Med Rehabil. 1986;67:169–171. doi: 10.1016/0003-9993(86)90064-x. [DOI] [PubMed] [Google Scholar]
- 28.Sun DY, Han J, Li XJ, Liu J, Song CL. Treatment of nine patients with amyotrophic lateral sclerosis by autologous peripheral blood mononuclear cells transplantation: change of short-term effect. Zhongguo Zuzhi Gongcheng Yanjiu. 2012;16:2645–2647. [Google Scholar]
- 29.Süssmuth SD, Tumani H, Ecker D, Ludolph AC. Amyotrophic lateral sclerosis: disease stage related changes of tau protein and S100 beta in cerebrospinal fluid and creatine kinase in serum. Neurosci Lett. 2003;353:57–60. doi: 10.1016/j.neulet.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 30.Tai H, Cui L, Liu M, Guan Y, Li X, Shen D, Zhang K, Liu S, Wu S, Ding Q, Hu Y. Creatine kinase level and its relationship with quantitative electromyographic characteristics in amyotrophic lateral sclerosis. Clin Neurophysiol. 2018;129:926–930. doi: 10.1016/j.clinph.2018.01.071. [DOI] [PubMed] [Google Scholar]
- 31.Tai H, Cui L, Guan Y, Liu M, Li X, Shen D, Li D, Cui B, Fang J, Ding Q, Zhang K, Liu S. Correlation of creatine kinase levels with clinical features and survival in amyotrophic lateral sclerosis. Front Neurol. 2017;8:322. doi: 10.3389/fneur.2017.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turner MR, Hardiman O, Benatar M, Brooks BR, Chio A, de Carvalho M, Ince PG, Lin C, Miller RG, Mitsumoto H, Nicholson G, Ravits J, Shaw PJ, Swash M, Talbot K, Traynor BJ, Van den Berg LH, Veldink JH, Vucic S, Kiernan MC. Controversies and priorities in amyotrophic lateral sclerosis. Lancet Neurol. 2013;12:310–322. doi: 10.1016/S1474-4422(13)70036-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei QQ, Chen Y, Chen X, Cao B, Ou R, Zhang L, Hou Y, Shang H. Prognostic nomogram associated with longer survival in amyotrophic lateral sclerosis patients. Aging Dis. 2018;9:965–975. doi: 10.14336/AD.2017.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.