Abstract
Substance use, specifically the use of prescription and non-prescription opioids among pregnant women, is a major public health issue and chief contributor to the opioid crisis. The prevalence of Neonatal Opioid Withdrawal Syndrome has risen 5-fold in the past decade, and is a well-recognized consequence of perinatal opioid exposure. By contrast, the long-term damage to the developing brain from opioid medications is just beginning to be recognized as a serious concern. Published data suggest that opioid exposure commencing in utero negatively affects the maturation of the neural-immune system, and trajectory of central nervous system development. Methadone induces peripheral immune hyper-reactivity, lasting structural and microstructural brain injury, and significant deficits in executive function and cognitive control in adult animals following in utero exposure. Thus, to address the cascading public health crisis stemming from the multitude of infants with in utero opioid exposure who will grow up with altered neurodevelopmental trajectories, rigorous preclinical, mechanistic studies are required. Such studies will define the long-term sequelae of prenatal opioid exposure in an effort to develop appropriate and targeted interventions. Specifically, the development of novel fluid, neuroimaging and biobehavioral biomarkers will be the most useful to aid in early identification and treatment of opioid exposed infants with the greatest risk of poor clinical outcomes. These studies will be essential to understand how in utero insults determine brain structure and function in adulthood, and what targeted interventions will be required to improve long-term outcomes in the countless children being born exposed to opioids each year.
Keywords: biomarker, buprenorphine, cognition, executive function, magnetic resonance imaging, methadone, neonate
Introduction
In the United States, the opioid crisis poses a severe threat to present and future public health. The acute emergency is increasingly apparent, with more than 100 US citizens dying from opioid overdoses every day (Hedegaard et al., 2018). Though this national crisis has received attention and the number of deaths secondary to opioid overdose is alarming, the full depth and breadth of the opioid crisis, especially the impact on infants and children, is often overlooked. Indeed, maternal opioid use rates during pregnancy have more than quadrupled in the last decade. According to the Centers for Disease Control, from 2008–2012, approximately 1 in every 3 pregnant women filled an opioid prescription. Opioid use in pregnancy entails the use and misuse of prescription opioids, such as codeine, morphine, oxycodone, and illicit opioids, such as heroin, or medications used to treat opioid use disorder, such as methadone and buprenorphine.
Among the limited attention given to perinatal opioid exposure, research has focused on the immediate perinatal effect from maternal opioid use, described as Neonatal Opioid Withdrawal Syndrome (NOWS) (Honein et al., 2019). The incidence of NOWS has increased five-fold since 2000 from 1.19 per 1000 hospital births in the US to 5.63 in 2012, while the incidence of infants treated in the NICU has increased five-fold in the same time period (Sanlorenzo et al., 2019). Although NOWS is a well-recognized consequence of perinatal opioid exposure, the full life-long impact of exposure, particularly on the developing brain and immune system is unknown (Patrick et al., 2017). Notably, the negative effects extend far beyond NOWS diagnoses and are apparent in the first days or weeks of life (Honein et al., 2019). While supportive and pharmacological therapies exist to manage opioid withdrawal in infants, their efficacy and long-term sequelae are controversial. Further, the opioids used to manage withdrawal symptoms are robustly proinflammatory (Jantzie et al., 2020). Thus, to address the cascading public health crisis stemming from the multitude of infants with in utero opioid exposure who will grow up with altered neurodevelopmental trajectories, rigorous preclinical, mechanistic studies are required. Such studies will define the long-term sequelae of prenatal opioid exposure in an effort to develop appropriate and targeted interventions. Similarly, the development of novel fluid, neuroimaging and biobehavioral biomarkers will be the most useful to aid in early identification and treatment of opioid exposed infants with the greatest risk of poor clinical outcomes.
The temporal effects of opioid exposure on the developing infant are unknown. This temporality is of extreme importance, especially from a public health standpoint. The effects of opioids on the developing brain and immune system might be subtle and long-lasting and cause permanent changes in immune function and brain structure and function (Figure 1). Opioids rapidly cross the placenta, and via the fetal circulation have a direct impact on developing organ systems, including the central nervous system. To this end, placental pathology may be vital. Placental and precision medicine starts in the neonatal intensive care unit (NICU). This is now true for many forms of perinatal brain injuries, including hypoxic-ischemic encephalopathy and encephalopathy of prematurity. The inclusion of placental examination in infants exposed to opioids may provide diagnostic avenues or aid in the stratification of neonates to emerging clinical trials for neurorepair. Previous studies emphasize the placenta as a source for individual-specific information on maternal and fetal health (Shallie and Naicker, 2019). However, to employ placental pathology in such precision medicine, time is a potential barrier to implementation unless histological information or molecular surrogate biomarkers are readily available. Despite this, placental pathology, including information on inflammatory infiltrate, acute and chronic inflammation and hypoxia/ischemia may allow for accelerated advances in biomarkers and treatment that would otherwise outweigh the time and cost disadvantages.
Figure 1.
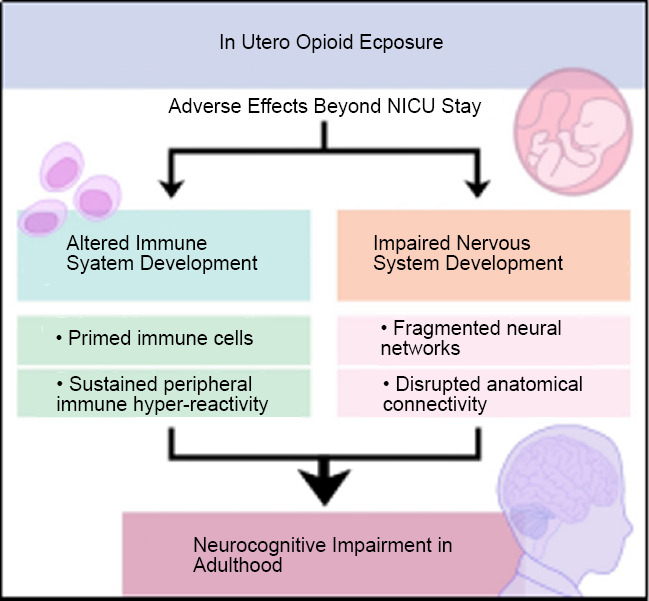
The origin of adult neurocognitive impairment associated with in utero opioid exposure is rooted in altered development of the immune and nervous systems.
Longitudinal Clinical Outcomes
Clinically, infants exposed to opioids can be discharged from hospitals after combating initial withdrawal symptoms. However, similar to extreme prematurity, perinatal stroke, and prenatal alcohol exposure, it is widely accepted that health problems for these children can arise later on in life. Children exposed to opioids during pregnancy are at an increased risk of neurodevelopmental impairment. Clinical investigations show children born to women who have been prescribed opioids, including methadone, are at higher risk of neurodevelopmental impairment (Monnelly et al., 2018, 2019), with lower Mental Development Index and Psychomotor Development Index scores than unexposed children. A recent systematic review and meta-analysis conducted by Lee et al. (2020) found that children who suffered perinatal opioid exposure are at high-risk of adverse neurodevelopment at least into middle childhood. There is some evidence of increased rate of diagnosis of attention deficit hyperactivity disorder among children aged 10–14 years who were prenatally exposed to opioids, with associated neuropsychological deficits on complex working memory tasks (Sirnes et al., 2018). Furthermore, these neuropsychological deficits were associated increased activation on functional MRI in the prefrontal cortices with increasing working memory demands. This is significant because the effects of perinatal opioid exposure might not manifest until children attend school. Recent preclinical studies have similarly shown significant deficits of cognitive control and executive function in juvenile rats following perinatal opioid exposure (Jantzie et al., 2020). The behavioral platform used in this study is comparable to the well-known CANTAB cognitive testing platform used in humans, and is directly translatable (Nithianantharajah and Grant, 2013). Additionally, these methadone-exposed rats demonstrated an inability to efficiently learn new associations and to reverse learned associations compared to unexposed controls. This upholds the notion that cognitive deficits may not be evident in the neonatal period, but can develop over time, and even emerge in adulthood. To this end, early triage to neurodevelopmental follow-up may be necessary in the NICU. Similarly, the development of advanced biobehavioral biomarkers that reflect the full clinical picture similar to those being used to diagnose infants with cerebral palsy may be paramount.
Neural Structural Modifications
Related to immediate identification and triage to neurodevelopmental follow-up, brain imaging in the NICU may be advantageous for the stratification, and potential treatment of infants exposed to opioids. Despite the limited number, clinical imaging studies have begun to demonstrate the negative impact of maternal opioid use on the developing brain. Structural MRI shows that infants exposed to opioids have significantly smaller brains and basal ganglia along with reduced cerebellar volumes compared to their non-exposed counterparts (Sirnes et al., 2017). Moreover, opioid-exposed infants have microstructural alterations in white matter tracts that can be identified at birth and throughout childhood using high-resolution diffusion sequences. In response to the recent demand that more animal studies be performed with controls for several of the confounding factors seen in human clinical studies of prenatal drug exposure (Skranes, 2019), we undertook a preclinical evaluation of perinatal methadone exposure in rats. We removed possible confounding variables such polysubstance use and abuse, and differing social factors (Jantzie et al., 2020). We were able to adequately model in rats the intact maternal-placental-fetal unit to precisely study opioid effects in depth. Similar to human neonates who suffered methadone exposure, our results revealed that rat pups of both sexes show a marked drop in body weight. Molecularly, we discovered that methadone exposure increases inflammatory cytokines in peripheral circulation and induces a robust, and sustained peripheral immune response concomitant with an increase in key cerebral molecular and cellular inflammatory mediators common to perinatal brain injury (Jantzie et al., 2020). Structurally in the brain, methadone also induced central nervous system injury. Methadone-exposed pups showed a significant reduction in axonal integrity and myelin expression, compared to rat pups exposed to saline. These data compliment previous preclinical investigations demonstrating that buprenorphine and methadone disrupt sequences of oligodendrocyte development and later timing of myelination (Sanchez et al., 2008; Eschenroeder et al., 2012; Vestal-Laborde et al., 2014; Oberoi et al., 2019). They also support the possibility of altered neurodevelopment induced by different protocols of experimental opioid exposure and support older literature suggesting detrimental effects of opioids on neuro-ontogeny and neural biochemistry (Zagon and McLaughlin, 1977; Robinson et al., 1997; Wu et al., 2001). Multi-modal imaging studies also reveal brain structure-function relationships that may be longitudinally relevant in prenatally opioid-exposed children. In adulthood, methadone exposed rat offspring have abnormal fractional anisotropy, disrupted microstructural integrity, and impaired structural coherence. These data fully align with clinical literature demonstrating a similar pattern of diffusion MRI abnormalities in children exposed to methadone in utero and in adults with chronic methadone exposure (Walhovd et al., 2012; Li et al., 2016; Monnelly et al., 2018).
While one can imagine a clinical algorithm involving placental pathology, neonatal MRI and long-term neurodevelopmental follow-up for infants exposed to opioids, a public health perspective demands a response based in prevention, reversal, treatment, and recovery services. Specific to women using opioids, improving access to treatment and increasing implementation of the spectrum of preventative strategies are of the utmost importance. However, for infants and children, a focus on tertiary prevention efforts is crucial. Indeed, tertiary prevention efforts are dedicated to improving long-term health outcomes. This is essential in the context of opioid exposure, as these children are at increased risk of neglect and abuse concomitant with cognitive and behavioral deficits that compound their lifetime risk of harm. Our goal is to elucidate the long-term health outcomes of prenatal opioid use, establishing a solid evidentiary base from which tertiary prevention efforts can expand.
Conclusion
In sum, there is an urgent need to not only define the full spectrum of adverse infant and childhood outcomes associated with perinatal opioid exposure, but to also develop novel therapies for opioid-exposed individuals. Biobehavioral, neuroimaging and liquid biomarkers of perinatal opioid induced-injury and repair will inform validation of treatment targets. Moreover, they will also inform diagnosis and future clinical trial design aimed at improving long-term outcomes. Thus, biomarkers in placenta and neonates could provide estimation of extent of immune system abnormalities and central nervous system injury, and provide pharmacodynamic support to guide duration or degree of treatment. In this context, a rigorous, complimentary, and coordinated approach to merging viable synergistic therapeutic strategies, with integration of neuroimaging and biobehavioral outcomes, is needed to transform the care of children exposed to opioids during development. Undoubtedly, the extent of perinatal opioid exposure, including dose, duration and cumulative effects is challenging to quantify. Clinically, opioid medications are recommended when supportive measures are insufficient to manage NOWS symptoms (Reddy et al., 2017). Opioids are also used routinely in NICUs for analgesia and in the context of interventions such as mechanical ventilation. Thus, further study is warranted to elaborate the full spectrum of their effects on brain development and long-term outcome, no matter the indication for or transient nature of their use.
Additional file: Open peer review report 1 (76.2KB, pdf) .
Footnotes
P-Reviewer: Li Y; C-Editors: Zhao M, Li JY; T-Editor: Jia Y Review
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Yunwei Li, Peking Union Medical College Hospital, China.
References
- 1.Eschenroeder AC, Vestal-Laborde AA, Sanchez ES, Robinson SE, Sato-Bigbee C. Oligodendrocyte responses to buprenorphine uncover novel and opposing roles of mu-opioid- and nociceptin/orphanin FQ receptors in cell development: implications for drug addiction treatment during pregnancy. Glia. 2012;60:125–136. doi: 10.1002/glia.21253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hedegaard H, Minino AM, Warner M. Drug overdose deaths in the United States, 1999-2017. NCHS Data Brief. 2018;2018:1–8. [PubMed] [Google Scholar]
- 3.Honein MA, Boyle C, Redfield RR. Public health surveillance of prenatal opioid exposure in mothers and infants. Pediatrics. 2019 doi: 10.1542/peds.2018-3801. doi: 101542/peds2018-3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jantzie LL, Maxwell JR, Newville JC, Yellowhair TR, Kitase Y, Madurai N, Ramachandra S, Bakhireva LN, Northington FJ, Gerner G, Tekes A, Milio LA, Brigman JL, Robinson S, Allan A. Prenatal opioid exposure: The next neonatal neuroinflammatory disease. Brain Behav Immun. 2020;84:45–58. doi: 10.1016/j.bbi.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SJ, Bora S, Austin NC, Westerman A, Henderson JMT. Neurodevelopmental outcomes of children born to opioid-dependent mothers: a systematic review and meta-analysis. Acad Pediatr. 2020;20:308–318. doi: 10.1016/j.acap.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Li W, Li Q, Wang Y, Zhu J, Ye J, Yan X, Li Y, Chen J, Liu J, Li Z, Wang W, Liu Y. Methadone-induced damage to white matter integrity in methadone maintenance patients: a longitudinal self-control DTI study. Sci Rep. 2016;6:19662. doi: 10.1038/srep19662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monnelly VJ, Anblagan D, Quigley A, Cabez MB, Cooper ES, Mactier H, Semple SI, Bastin ME, Boardman JP. Prenatal methadone exposure is associated with altered neonatal brain development. Neuroimage Clin. 2018;18:9–14. doi: 10.1016/j.nicl.2017.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monnelly VJ, Hamilton R, Chappell FM, Mactier H, Boardman JP. Childhood neurodevelopment after prescription of maintenance methadone for opioid dependency in pregnancy: a systematic review and meta-analysis. Dev Med Child Neurol. 2019;61:750–760. doi: 10.1111/dmcn.14117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nithianantharajah J, Grant SG. Cognitive components in mice and humans: combining genetics and touchscreens for medical translation. Neurobiol Learn Mem. 2013;105:13–19. doi: 10.1016/j.nlm.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Oberoi R, Chu T, Mellen N, Jagadapillai R, Ouyang H, Devlin LA, Cai J. Diverse changes in myelin protein expression in rat brain after perinatal methadone exposure. Acta Neurobiol Exp (Wars) 2019;79:367–373. [PubMed] [Google Scholar]
- 11.Patrick SW, Schiff DM Committee On Substance Use and Prevention. Pediatrics. 2017 doi: 101542/peds2016-4070. [Google Scholar]
- 12.Reddy UM, Davis JM, Ren Z, Greene MF Opioid Use in Pregnancy NAS, Childhood Outcomes Workshop Invited S. Opioid use in pregnancy, Neonatal Abstinence Syndrome, and Childhood Outcomes: Executive Summary of a Joint Workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, American College of Obstetricians and Gynecologists, American Academy of Pediatrics, Society for Maternal-Fetal Medicine, Centers for Disease Control and Prevention, and the March of Dimes Foundation. Obstet Gynecol. 2017;130:10–28. doi: 10.1097/AOG.0000000000002054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson SE, Maher JR, Wallace MJ, Kunko PM. Perinatal methadone exposure affects dopamine, norepinephrine, and serotonin in the weanling rat. Neurotoxicol Teratol. 1997;19:295–303. doi: 10.1016/s0892-0362(97)00018-4. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez ES, Bigbee JW, Fobbs W, Robinson SE, Sato-Bigbee C. Opioid addiction and pregnancy: perinatal exposure to buprenorphine affects myelination in the developing brain. Glia. 2008;56:1017–1027. doi: 10.1002/glia.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanlorenzo LA, Stark AR, Patrick SW. Neonatal abstinence syndrome: an update. Curr Opin Pediatr. 2018;30:182–186. doi: 10.1097/MOP.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shallie PD, Naicker T. The placenta as a window to the brain: A review on the role of placental markers in prenatal programming of neurodevelopment. Int J Dev Neurosci. 2019;73:41–49. doi: 10.1016/j.ijdevneu.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Sirnes E, Griffiths ST, Aukland SM, Eide GE, Elgen IB, Gundersen H. Functional MRI in prenatally opioid-exposed children during a working memory-selective attention task. Neurotoxicol Teratol. 2018;66:46–54. doi: 10.1016/j.ntt.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Sirnes E, Oltedal L, Bartsch H, Eide GE, Elgen IB, Aukland SM. Brain morphology in school-aged children with prenatal opioid exposure: A structural MRI study. Early Hum Dev. 2017;106-107:33–39. doi: 10.1016/j.earlhumdev.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Skranes J. Children exposed to maternal methadone treatment prenatally are at risk of abnormal neurodevelopment. Dev Med Child Neurol. 2019;61:738. doi: 10.1111/dmcn.14130. [DOI] [PubMed] [Google Scholar]
- 20.Vestal-Laborde AA, Eschenroeder AC, Bigbee JW, Robinson SE, Sato-Bigbee C. The opioid system and brain development: effects of methadone on the oligodendrocyte lineage and the early stages of myelination. Dev Neurosci. 2014;36:409–421. doi: 10.1159/000365074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walhovd KB, Watts R, Amlien I, Woodward LJ. Neural tract development of infants born to methadone-maintained mothers. Pediatr Neurol. 2012;47:1–6. doi: 10.1016/j.pediatrneurol.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Wu VW, Mo Q, Yabe T, Schwartz JP, Robinson SE. Perinatal opioids reduce striatal nerve growth factor content in rat striatum. Eur J Pharmacol. 2001;414:211–214. doi: 10.1016/s0014-2999(01)00807-x. [DOI] [PubMed] [Google Scholar]
- 23.Zagon IS, McLaughlin PJ. The effects of different schedules of methadone treatment on rat brain development. Exp Neurol. 1977;56:538–552. doi: 10.1016/0014-4886(77)90320-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


