Figure 1.
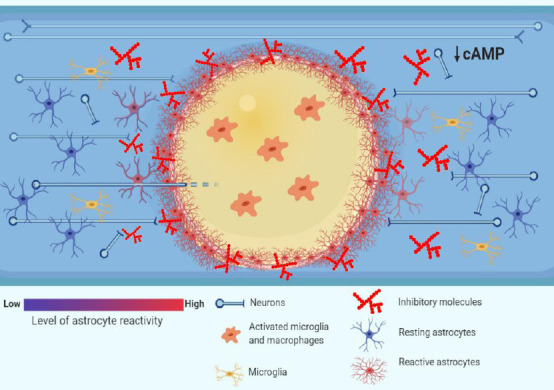
Pathophysiology of traumatic spinal cord injury (TSCI): barriers to axonal regrowth.
Following TSCI, the resulting ischemia, hemorrhage and inflammation generate zones of dying tissue that, with time, transform into cystic cavities (yellow zone in the picture). The cytotoxic environment in the cavities, which are filled with activated inflammatory cells such as microglia and macrophages, renders them a poor substrate to axonal regrowth (O’Shea et al., 2017). The cystic cavities are encircled by a glial scar that is formed by reactive astrocytes. The astrocytes undergo hypertrophy and wall off the cytotoxic environment of the cysts from the spared neural tissue, at the same time forming a physical barrier to axonal regrowth (Sofroniew, 2014). Moreover, the chemical barrier to axonal regrowth is set up by activated glial cells such as astrocytes, macrophages and oligodendrocyte progenitor cells (OPCs), which secrete inhibitory molecules such as chondroitin sulphate proteoglycans (CSPGs) (Busch and Silver, 2007). Finally, the intrinsic abilities of axons to regrow are undermined by low levels of cyclic adenosine 3′,5′-monophosphate (cAMP), which drop postnatally and decrease further after spinal cord injury (Pearse et al., 2004), and which govern a response of growth cones to extracellular guidance molecules such as myelin-associated glycoprotein (MAG) (Peace and Shewan, 2011).
