Abstract
Background
The rapid Severe Acute Respiratory Syndrome Coronavirus 2 (SARS CoV2) outbreak caused severe pandemic infection worldwide. The high mortality and morbidity rate of SARS CoV2 is due to the unavailability of vaccination and mutation in this virus. The present article aims to design a potential vaccine construct VTC3 targeting the non-mutational region of structural and non-structural proteins of SARS CoV2.
Methods
In this study, vaccines were designed using subtractive proteomics and reverse vaccinology. To target the virus adhesion and evasion, 10 different structural and non-structural proteins have been selected. Shortlisted proteins have been screened for B cell, T cell and IFN gamma interacting epitopes. 3D structure of vaccine construct was modeled and evaluated for its physicochemical properties, immunogenicity, allergenicity, toxicity and antigenicity. The finalized construct was implemented for docking and molecular dynamics simulation (MDS) with different toll-like receptors (TLRs) and human leukocyte antigen (HLA). The binding energy and dissociation construct of the vaccine with HLA and TLR was also calculated. Mutational sensitivity profiling of the designed vaccine was performed, and mutations were reconfirmed from the experimental database. Antibody production, clonal selection, antigen processing, immune response and memory generation in host cells after injection of the vaccine was also monitored using immune simulation.
Results
Subtractive proteomics identified seven (structural and non-structural) proteins of this virus that have a role in cell adhesion and infection. The different epitopes were predicted, and only extracellular epitopes were selected that do not have similarity and cross-reactivity with the host cell. Finalized epitopes of all proteins with minimum allergenicity and toxicity were joined using linkers to designed different vaccine constructs. Docking different constructs with different TLRs and HLA demonstrated a stable and reliable binding affinity of VTC3 with the TLRs and HLAs. MDS analysis further confirms the interaction of VTC3 with HLA and TLR1/2 complex. The VTC3 has a favorable binding affinity and dissociation constant with HLA and TLR. The VTC3 does not have similarities with the human microbiome, and most of the interacting residues of VTC3 do not have mutations. The immune simulation result showed that VTC3 induces a strong immune response. The present study designs a multiepitope vaccine targeting the non-mutational region of structural and non-structural proteins of the SARS CoV2 using an immunoinformatic approach, which needs to be experimentally validated.
Keywords: Multiepitope vaccine, SARS CoV2, Membrane proteins, ORF8 protein, ORF3a protein, Envelope protein, Surface glycoprotein, ORF1ab polyprotein
Introduction
Immuno-pathogenesis of the infectious epidemic COVID-19 emerged as serious intimidation worldwide. In the Coronaviridae family, among four different coronavirus classes (alpha, beta, delta and gamma), alpha and beta positive-sense RNA virus strains have been confirmed for broadly epidemic infection (De Wilde et al., 2018; Tahir ul Qamar et al., 2020). Severe Acute Respiratory Syndrome Coronavirus 2 (SARS CoV2) mediated outbreak of the disease was firstly reported in Wuhan city, China, in December 2019 (Gorbalenya et al., 2020; Huang et al., 2020; Perlman, 2020; Tahir ul Qamar et al., 2019; World Health Organization, 2020; Wu, Leung & Leung, 2020; Zhu et al., 2020). The outbreak has so far infected more than 10,00,000 patients worldwide on dated 10 May 2020. SARS CoV2 genome sequence comparison showed almost 96%, 79.5% and 40% similarities with bat coronavirus, SARS CoV and MERS CoV strain, respectively (Alamri, Tahir ul Qamar & Alqahtani, 2020; Benvenuto et al., 2020; Zhou et al., 2020). The clinical symptoms of SARS CoV2 exhibit up to 14 days in infected people with fever (≥38 °C), diarrhea, dry cough, low peripheral WBC count, respiratory disorder and low lymphocyte count (Huang et al., 2020).
Among SARS CoV2 genomic RNA, 66% codes for the non-structural or essential proteins, and 33% codes for the structural or accessory proteins. Non-structural proteins have a significant role in virus replication and transcription in the host cell, while structural proteins cover the outer surface and participate in the host evasion system to cause the infection. SARS CoV2 mutation prone (Jiayuan et al., 2020) genomic organization is a difficult task to develop a vaccine that is composed of 5′-leader-UTR-replicase-ORFab-S(Spike)-E(Envelope)-M(Membrane)-ORF6-ORF7a-ORF8-N(Nucleocapsid)-3′UTR-polyA tail. Accessory proteins like ORF3a, ORF7a, and ORF8 are essential for viral pathogenesis (Seema, 2020; Zhu et al., 2020).
To overcome the issues such as cost and time associated with traditional method (monoclonal oligonucleotides, small drug molecules), of disease prevention (Li & De Clercq, 2020), the virus was analyzed by computational methods (Chen, Liu & Guo, 2020). Recently published immuno-informatics based vaccine design against MERS virus, Ebola virus chikungunya, and Zika showed the promising potential to fight against disease (Ahmad et al., 2019; Shahid et al., 2020; Tahir ul Qamar et al., 2018, 2019). Reverse vaccinology includes different software algorithms to evaluate the immunological data that analyze the epitopes binding efficiency with human leukocyte antigen (HLA) alleles, antigenicity, allergenicity and toxicity to design the potential multiepitope subunit vaccine (De Gregorio & Rappuoli, 2012; Khan et al., 2018; Mirza et al., 2016; Patronov & Doytchinova, 2013). The final multiepitope vaccine is a group of different epitopes joined with the help of linkers that may enhance specific adaptive-immune responses in the host cell (Brennick et al., 2017; Chauhan et al., 2019; Jensen & Andreatta, 2018; Lu & Meng, 2017; Nain et al., 2020; Purcell, McCluskey & Rossjohn, 2007; Saadi, Karkhah & Nouri, 2017). To activate the host immune system, interaction of toll-like receptors (TLRs) with the designed vaccine could be a potential approach to combat viral infection. Intracellular TLRs that present on cell endosomes interact with ssRNA (TLR-7 and TLR-8), dsRNA (TLR-3) and CpG DNA (TLR-9) to activate the NFkβ mediated immune/cell response (Carty & Bowie, 2010). With protein-based epitope vaccine designing, TLR-2, TLR-4, TLR2/6 and TLR1/2 heterodimer mediated immune cell activation (IRF3/7 and NFKβ) is an essential factor to activate the innate immunity. Basal TLR-2 and TLR4 activation needs for host protective immunity, whereas its overexpression leads to subvert effect by inducing the viral cell replication in the host cell (Olejnik, Hume & Muhlberger, 2018); hence, it is better to target TLR1/2 heterodimer mediated activation signaling with mild TLR-4 interaction/activation (Olejnik, Hume & Muhlberger, 2018).
In the present study, non-mutational sequences of SARS-CoV2 were targeted to design a multiepitope vaccine construct. SARS-CoV2 proteome was investigated to identify antigenic proteins and different T-cell and B-cell epitopes were identified in selected proteins, and their antigenicity, allergenicity and physiochemical properties have been evaluated. The molecular docking of final epitope constructs was performed with different TLRs and HLA alleles to confirm the stable binding interaction of the multi-epitope vaccine-receptor complex. Vaccine constructs were further assessed for its cross-reactivity and immune response in humans, as well as their similarity with the human microbiome.
Materials and Methods
Protein sequence collection
To evaluate the coronavirus suitable antigenic vaccine target, the different protein sequences have been retrieved from NCBI (https://www.ncbi.nlm.nih.gov/). These proteins are ORF6 protein (YP_009724394.1), membrane glycoprotein (YP_009724393.1), ORF3a protein (YP_009724391.1), nucleocapsid phosphoprotein (YP_009724397.2), ORF10 protein (QHI42199.1), ORF7a protein (YP_009724395.1), envelope protein (YP_009724392.1), ORF1ab (YP_009724389.1), ORF8 protein (QHD43422.1) and surface glycoprotein (YP_009724390.1). All FASTA sequences were used as an input for further immuno-informatics analysis (Seema, 2020).
Analysis of protein antigenicity and trans-membrane helicity
In developing the chimeric multi-epitope vaccine, protein antigenicity and transmembrane helicity play a key role in vaccine successes. To evaluate the protein antigenicity, the Vaxijen server (Doytchinova & Flower, 2007) with 0.4 thresholds has been used, while trans-membrane helicity was estimated using TMHMM (Krogh et al., 2001) and Protter server (Omasits et al., 2014).
Cytotoxic T cell epitopes prediction with potential antigenicity, allergenicity and Toxicity
Shortlisted seven extracellular protein peptides were evaluated by NetCTLpan version 1.1 to predict promiscuous epitopes that can enhance the host cell’s immune response by interacting with HLA-epitope binding. NetCTLpan analysis was performed for all MHC (HLA) class I molecules supertype representative (HLA-A01:01, HLA-A02:01, HLA-A03:01, HLA-A24:02, HLA-A26:01, HLA-B07:02, HLA-B08:01, HLA-B27:05, HLA-B39:01, HLA-B40:01, HLA-B58:01, HLA-B15:01), and predicts the 8, 9, 10 and 11 mer peptides to reduce the experimental efforts. With the help of the neural network, the algorithm server has predicted promiscuous high binding affinity nonameric peptides (Stranzl et al., 2010). HLA binder peptides were further evaluated by Vaxijen (http://www.ddg-pharmfac.net/vaxijen/VaxiJen/VaxiJen.html). AllergenFP (Dimitrov et al., 2013) and Toxinpred server to confirm the epitope’s antigenicity, allergenicity and toxicity level, respectively (Gupta et al., 2013).
Immunogenicity prediction
Immunogenicity prediction (IEDB) confers the property which can elicit the cellular and humoral response in the host cell against viral infection. Shortlisted NetCTLpan epitopes were used for immunogenicity prediction that confers the property which can elicit the cellular and humoral response in the host cell against viral infection. Promiscuous epitopes ORF3a protein (one epitope), surface glycoprotein (two epitopes) and ORF1ab polyprotein (17 epitopes) were used as input peptides for immunogenicity prediction. High binding capacity epitopes (by IEDB immunogenicity tool server) were selected as positive immunogenicity epitopes. This server predicted the immunogenicity level based on the epitope positions in the expected peptide and physicochemical properties of an amino acid (Calis et al., 2013).
Helper T cell epitope prediction
With the help of the IEDB MHC II binding prediction tool, T helper cell epitopes were predicted. In vaccine development, the vaccine must cover most of the world’s population. Hence to overcome this HLA alleles variation, a reference set has been used that includes the panel of 27 alleles, which covers the >99% of the population (Greenbaum et al., 2011). IEDB MHC II server is based on a combinational library that generates the percentile rank and IC-50. The lower percentile rank represents a higher affinity of the epitope-HLA complex. The epitope-alleles affinity consensus list was generated for 15 amino acids long epitopes (Wang et al., 2008).
B cell epitope prediction
B cell epitopes detect viral infections by an antibody-based immune response. IEDB BEPIPRED (Jespersen et al., 2017) and ABCpred (Saha & Raghava, 2006) was used to analyze the B cell interacting epitopes. Overlapped epitopes from both servers were chosen for further analysis. FASTA files of all seven proteins were used as an input file. These resultant epitopes were further screened by Vaxijen, AllergenFP and TOXINPRED server to analyze the epitope’s antigenicity, allergenicity and toxicity reaction in the host cell.
Comparative cross-reactivity, IFN gamma induction analysis of MHC I, II and B cell epitopes
The comparative evaluation of all the finalized MHC I, MHC II and B cell epitopes has been done to remove the overlapping epitopes. Non-overlapping epitopes should have a unique presence in the virus. To minimize the cross-reactivity in host cells, non-overlapping epitopes were BLAST against human proteome (ID 9606) using Protein Information Resource (Chen et al., 2013). Besides, IFN gamma induction has a vital role in viral elimination and host immune response induction. Hence, we have predicted the IFN gamma induction efficiency of selected non-cross-reactive epitopes via the IFNepitope server (http://crdd.osdd.net/raghava/ifnepitope/).
Multi-epitope vaccine designing
All screened epitopes that contain the antigenic property with no allergenicity, toxicity and cross-reactivity have been finalized to design the chimeric multi-epitope vaccine construct against SARS CoV2. A potent vaccine should activate the immune response in the host cell but not activates the detrimental immunity. Hence, to maintain the balance of immunity in the host cell, half non-IFN gamma inducible peptides have been selected, and vise-versa. Epitope binding with B and T cell receptor needs a degree of freedom. Based on the linker’s length, flexibility and rigidity, EAAAK and GGGS linker have been used. EAAAK linkers rigidity is used to maintain spaces between proteins domain while GGGS linkers provide flexibility to construct with minimum linker-epitope interference to maintain the epitope function (Solanki & Tiwari, 2018).
Evaluation of potential vaccine candidate or construct
Vaccine constructs binding affinity with B and T cell, IgE antibody-associated allergic reaction and post-injection toxicity to host cell are a significant issue in vaccine development. Hence, different vaccine constructs were further investigated for antigenicity (via Vaxijen), allergenicity (via AllergenFP) and toxicity (via Toxinpred). Vaccine constructs physicochemical behavior analysis is the major key to find out the post-injection symptoms. A highly antigenic vaccine construct (i.e., VTC3) with lower allergic and toxicity has been further evaluated by the Protparam Server (Gasteiger et al., 2003). The web server provides molecular weight (KDa), theoretical isoelectric point (pI), half-life, stability index, aliphatic index, extinction coefficient and grand average of hydropathicity (GRAVY). The secondary and tertiary structure of selected vaccine construct VTC3 was predicted using PESIPRED (Buchan & Jones, 2019) and Phyre2 in intensive modeling mode (Kelley et al., 2015) respectively. To further cross-validate the model structure, ab-initio modeling of the vaccine construct was performed by RaptorX (http://raptorx.uchicago.edu). The modeled vaccine was refined by GalaxyRefine server and validated by PSVS analysis, ProSA (Wiederstein & Sippl, 2007) and QMEAN (Studer et al., 2019) per published protocol.
Molecular docking
The vaccine should induce cell-mediated and humoral immune responses in the host cell to eliminate the virus infection. VTC3 binding studies were performed with TLRs (TLR1, TLR2, TLR3, TLR4, TLR1/2, TLR6) and HLA alleles via molecular docking using PatchDock server (Schneidman-Duhovny et al., 2005). The docking was further validated by the HDOCK server (http://hdock.phys.hust.edu.cn/) (Yan et al., 2020)
Molecular dynamics simulation
The Molecular Dynamics Simulation (MDS) analysis was performed by Desmond using the OPLS3e force field and TIP3P solvent. MDS was run for 50ns in duplicate as per published methods (Tiwari, 2021). In brief, the system was built by selecting TIP3P water as a solvent; the system’s charge was neutralized by adding twenty-nine sodium ions, the system was made close to the natural system by adding 0.15M NaCl. The build system was relaxed before simulation, and MDS was performed for 50 ns. The MDS trajectories were recorded and analyzed for RMSD and RMSF.
Binding affinity and dissociation constant calculation
The binding affinity as well as dissociation constant of the VTC3 vaccine construct with the HLA and TLRs were further estimated at different simulation time periods using the PRODIGY server (Vangone & Bonvin, 2017). The MDS trajectories at different time intervals were saved in PDB format and used to calculate the binding affinity and dissociation constant.
Validation of potential vaccine candidate or construct with the human microbiome
Potential vaccine candidate sequence similarity with the gut microflora would unintentionally interfere with the proteins of the host’s microflora. To minimize this adverse effect, vaccine candidate VTC3 was BLAST against proteomes of gut flora (226 organisms) (Ramos et al., 2018) identified in the Human Microbiome Project (Peterson et al., 2009). The BLAST was performed with a significant hit (E-value ≤ 10−5) and identity ≥40% (Ramos et al., 2018). For further target prioritization, hits with identity ≥40% with a human protein were filtered out, because it could have cross-interference if the virus protein were used as a target.
Mutational sensitivity profiling of VTC3 and experimental mutation database analysis
MAESTRO web server (Laimer et al., 2016) was used for mutational sensitivity profiling of designed multiepitope vaccine construct VTC3. The amino acid sequence of VTC3 was further analyzed for any mutation present in the SARS CoV2 virus with the help of the CoV-GLUE database (update June 2020) using published methods (Tiwari, 2020b).
Immune simulations
Multi epitope vaccine immune simulation profile was performed using C-ImmSim online server (http://kraken.iac.rm.cnr.it/C-IMMSIM/). This server is based on the Celada-Seiden model that predicts the immune (humoral and cellular) stimulation against vaccine construct (Rapin et al., 2010). The simulation was performed with default parameters like random seed 12,345, simulation volume 10, simulation steps 672, and vaccine injection with no LPS. The parameters were used for vaccine injection with selected MHCs (A0101, B5802, B4403, DRB3_0101, DRB1_0101) and 1,000 antigens. The VTC3 vaccine (no LPS) injection was given on 1st day, 4 weeks and 16 weeks (equivalent time steps is 1, 84,336 steps, respectively, where each time step is 8 h). To further assess the virus clearance capacity of the active immune system, the same antigen is used as a virus and observed that virus elimination. The virus was injected at 24 weeks (504 steps) with multiplication factor 0.2, infectivity 0.6 and virus number of 1,000. The different immune responses in the host after injection of VTC3 and virus were analyzed from the different plots.
Codon adaptation for vaccine construct and in-silico cloning
The amino acid sequence of VTC3 was reverse translated to DNA. Codon optimization is important for cloning and expression of VTC3 genes. The Java Codon Adaptation tool (http://www.jcat.de/) was utilized for codon optimization of VTC3 genes in a prokaryotic organism like E. coli. The E. coli K12 strain was selected to enhance the expression efficiency of VTC3. The rho-independent transcription terminators, prokaryotic ribosome binding sites, and cleavage sites of some restriction enzymes were avoided. Sites for restriction endonuclease HindIII and BamHI were added to the N and C terminals of the VTC3 gene, respectively, and subsequently cloned between HindIII and BamHI site of pET28a (+) vector using the SnapGene 4.2 tool (https:/snapgene.com/) to ensure the in-vitro expression.
Result
Analysis of protein antigenicity and trans-membrane helicity
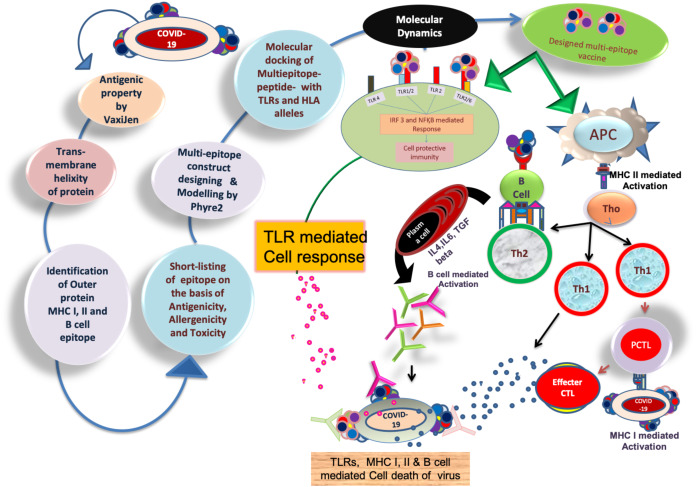
The workflow has been discussed in Fig. 1, which explains the reverse vaccinology mechanism used in the present study. Amino acid sequences of target proteins were collected in FASTA format to analyse antigenicity and trans-membrane helicity. All the proteins with their antigenicity score such as nucleoprotein phosphoprotein (0.50), ORF10 protein (0.71), ORF8 protein (0.65), ORF7a protein (0.64), ORF6 protein (0.61), membrane glycoprotein (0.51), an envelope protein (0.60), ORF3a protein (0.49), surface glycoprotein (0.46) and ORF1ab protein (0.46) showed their potential to exceed the immune response in the host cell. To elicit the host cell’s immune response, the transmembrane helicity of viral proteins has been evaluated. With the help of TMHMM and Protter servers, we have identified the extracellular, transmembrane and cytosolic peptides. The protein protter results showed that nucleoprotein phosphoprotein, ORF6 protein, and ORF10 protein are completely cytosolic. Hence, for further study, these three proteins were eliminated, and the rest of the seven proteins were considered for further study in designing the chimeric vaccine against SARS CoV2. The extracellular peptides of these proteins would help to interact with host PAMPs and maximize the solubility of designed vaccines.
Figure 1. Brief workflow of combinational chimeric multi-epitope vaccine designing with predicted immune cell response.
Cytotoxic T cell epitopes prediction with potential antigenicity, no allergenicity and toxicity
Cytotoxic T lymphocytes epitope predictions of all seven proteins were made using NetCTLpan 1.1 using the same HLA supertypes. Out of all seven, membrane glycoprotein (YP_009724393.1) did not get any potent HLA binder peptide. The rest six proteins showed the peptide binders with different HLA supertypes. From this server results, we have identified different epitopes ORF8 (eight epitopes), ORF7a protein (nine epitopes), envelope protein (four epitopes), ORF3a protein (three epitopes), surface glycoprotein (125 epitopes) and ORF1ab polyprotein (707 epitopes) binders. From the results, we manually screened peptides that showed a binding affinity with more than one HLA allele. This approach will minimize the HLA polymorphism so that promiscuous peptides will show binding to HLA of the wide population. After the manual screening of proteins, we have shortlisted ORF8 (one epitope), ORF7a protein (two epitopes), envelope protein (two epitopes), ORF3a protein (three epitopes), surface glycoprotein (43 epitopes) and ORF1ab polyprotein (277 epitopes) epitope binders. Based on antigenicity, allergenicity and toxicity analysis of peptide, different epitopes of ORF8 (zero epitopes), ORF7a protein (zero epitopes), envelope protein (zero epitopes), ORF3a protein (one epitope), surface glycoprotein (two epitopes) and ORF1ab polyprotein (17 epitopes) were further shortlisted, and the potential epitopes were selected that can enhance immune response via HLA activation (Table 1).
Table 1. MHC I binder epitopes showing result of antigenicity, allergenicity, toxicity and immunogenicity analysis.
| S. no | Protein name | Start | Epitope | Antigenicity | Allergenicity | Toxicity | Immunogenicity |
|---|---|---|---|---|---|---|---|
| 1 | ORF3a | 4 | MRIFTIGTV | 0.69 | Non-allergenic | Non-toxic | 0.37 |
| 2 | Surface glycoprotein | 242 | WTAGAAAYY | 0.63 | Non-allergenic | Non-toxic | 0.15 |
| 702 | FTISVTTEI | 0.85 | Non-allergenic | Non-toxic | −0.18 | ||
| 3 | Orf1ab polyprotein | 3449 | LSFKELLVY | 0.72 | Non-allergenic | Non-toxic | −0.07 |
| 1890 | EIDPKLDNY | 1.61 | Non-allergenic | Non-toxic | −0.2 | ||
| 1502 | ETISLAGSY | 0.59 | Non-allergenic | Non-toxic | −0.16 | ||
| 3841 | LSDDAVVCF | 0.58 | Non-allergenic | Non-toxic | 0.1 | ||
| 5467 | YTEISFMLW | 1.21 | Non-allergenic | Non-toxic | −0.03 | ||
| 2413 | VVTTFDSEY | 0.45 | Non-allergenic | Non-toxic | 0.1 | ||
| 295 | FMGRIRSVY | 0.52 | Non-allergenic | Non-toxic | 0.125 | ||
| 3471 | LLDKRTTCF | 1.76 | Non-allergenic | Non-toxic | −0.12 | ||
| 4672 | SMMGFKMNY | 1.3 | Non-allergenic | Non-toxic | −0.26 | ||
| 4615 | LQAENVTGL | 0.82 | Non-allergenic | Non-toxic | 0.19 | ||
| 2166 | NYMPYFFTL | 1 | Non-allergenic | Non-toxic | 0.15 | ||
| 5726 | IQLSSYSLF | 0.75 | Non-allergenic | Non-toxic | −0.48 | ||
| 524 | EQKSILSPL | 0.55 | Non-allergenic | Non-toxic | −0.26 | ||
| 5406 | FELEDFIPM | 1.26 | Non-allergenic | Non-toxic | 0.33 | ||
| 724 | EETGLLMPL | 0.48 | Non-allergenic | Non-toxic | −0.12 | ||
| 4055 | KLVLSVNPY | 0.54 | Non-allergenic | Non-toxic | −0.13 |
Immunogenicity prediction
Immunogenicity prediction of ORF3a protein (one epitope), surface glycoprotein (two epitopes) and ORF1ab polyprotein (17 epitopes) epitopes had shown the positive immunogenicity score of ORF3a protein (one epitope), surface glycoprotein (one epitope) and ORF1ab polyprotein (six epitopes) epitopes (Table 1). The positive immunogenicity with HLA binding confirmed that these epitopes would elicit a high immune response.
Helper T cell epitopes prediction with antigenicity, no allergenicity and toxicity
Helper T-cell mediated 15 amino acid extended epitopes were generated against the HLA allele reference set. In results, the percentile rank 0.1 was set as the threshold to filter the HLA interacting epitopes binding affinity. Based on reference threshold, ORF8 (five epitopes), ORF7a protein (zero epitopes), membrane protein (zero epitopes), envelope protein (zero epitopes), ORF3a protein (zero epitopes), surface glycoprotein (19 epitopes) and ORF1ab polyprotein (18 epitopes) showed <0.1 percentile rank (Table 2). Low percentile epitopes antigenicity (Table 2), allergenicity and toxicity level (Table 3) further shortlisted the epitopes. The compiled results of all the analyses identified surface glycoprotein (12 epitopes), ORF1ab polyprotein (four epitopes) and ORF8 (three epitopes) as HLA-II binders with high antigenicity booster and minimum allergenicity and toxicity.
Table 2. MHC II peptide showing percentile rank, and antigenicity score.
| S. no | Protein name | Allele | Start | Peptide | Percentile_rank | Antigenicity |
|---|---|---|---|---|---|---|
| 1 | ORF8 protein | HLA-DRB3*01:01 | 14 | QPYVVDDPCPIHFYS | 0.07 | 0.4574 |
| HLA-DRB3*01:01 | 10 | CTQHQPYVVDDPCPI | 0.08 | 0.5165 | ||
| HLA-DRB3*01:01 | 13 | HQPYVVDDPCPIHFY | 0.08 | 0.55 | ||
| HLA-DRB3*01:01 | 12 | QHQPYVVDDPCPIHF | 0.08 | 0.8637 | ||
| HLA-DRB3*01:01 | 11 | TQHQPYVVDDPCPIH | 0.08 | 0.6706 | ||
| 2 | Surface glycoprotein | HLA-DRB1*13:02 | 98 | KTQSLLIVNNATNVV | 0.01 | 0.63 |
| HLA-DRB1*13:02 | 102 | LLIVNNATNVVIKVC | 0.01 | 0.09 | ||
| HLA-DRB1*13:02 | 100 | QSLLIVNNATNVVIK | 0.01 | 0.43 | ||
| HLA-DRB1*13:02 | 101 | SLLIVNNATNVVIKV | 0.01 | 0.47 | ||
| HLA-DRB1*13:02 | 99 | TQSLLIVNNATNVVI | 0.01 | 0.43 | ||
| HLA-DRB3*02:02 | 100 | QSLLIVNNATNVVIK | 0.02 | 0.43 | ||
| HLA-DPA1*01:03/DPB1*04:01 | 323 | FGEVFNATRFASVYA | 0.03 | 0.04 | ||
| HLA-DRB1*01:01 | 498 | LSFELLHAPATVCGP | 0.03 | 0.5 | ||
| HLA-DRB1*01:01 | 497 | VLSFELLHAPATVCG | 0.03 | 0.47 | ||
| HLA-DRB1*01:01 | 496 | VVLSFELLHAPATVC | 0.03 | 0.86 | ||
| HLA-DRB1*13:02 | 103 | LIVNNATNVVIKVCE | 0.03 | −0.11 | ||
| HLA-DRB1*13:02 | 97 | SKTQSLLIVNNATNV | 0.03 | 0.62 | ||
| HLA-DRB3*02:02 | 101 | SLLIVNNATNVVIKV | 0.03 | 0.47 | ||
| HLA-DRB3*02:02 | 99 | TQSLLIVNNATNVVI | 0.06 | 0.433 | ||
| HLA-DPA1*01:03/DPB1*04:01 | 324 | GEVFNATRFASVYAW | 0.07 | −0.12 | ||
| HLA-DPA1*01:03/DPB1*04:01 | 322 | PFGEVFNATRFASVY | 0.07 | 0.03 | ||
| HLA-DRB1*01:01 | 499 | SFELLHAPATVCGPK | 0.09 | 0.2 | ||
| HLA-DRB1*01:01 | 495 | VVVLSFELLHAPATV | 0.09 | 0.8 | ||
| HLA-DRB3*02:02 | 102 | LLIVNNATNVVIKVC | 0.09 | 0.09 | ||
| HLA-DRB1*13:02 | 98 | KTQSLLIVNNATNVV | 0.01 | 0.63 | ||
| HLA-DRB1*13:02 | 102 | LLIVNNATNVVIKVC | 0.01 | 0.09 | ||
| 3 | Orf1ab polyprotein | HLA-DRB1*09:01 | 54 | AIILASFSASTSAFV | 0.01 | 0.23 |
| HLA-DQA1*01:02/DQB1*06:02 | 45 | AFASEAARVVRSIFS | 0.08 | −0.02 | ||
| HLA-DRB1*07:01 | 10 | CTFTRSTNSRIKASM | 0.12 | −0.02 | ||
| HLA-DPA1*03:01/DPB1*04:02 | 1 | YFFTLLLQLCTFTRS | 0.16 | −0.37 | ||
| HLA-DRB5*01:01 | 33 | LGRYMSALNHTKKWK | 0.17 | 0.04 | ||
| HLA-DRB1*01:01 | 20 | KSAFYILPSIISNEK | 0.27 | 0.71 | ||
| HLA-DPA1*03:01/DPB1*04:02 | 2165 | CTNYMPYFFTLLLQL | 0.01 | 0.45 | ||
| HLA-DRB1*01:01 | 1801 | ESPFVMMSAPPAQYE | 0.01 | 0.54 | ||
| HLA-DRB1*09:01 | 474 | AIILASFSASTSAFV | 0.01 | 0.23 | ||
| HLA-DPA1*03:01/DPB1*04:02 | 1244 | EETKFLTENLLLYID | 0.03 | −0.11 | ||
| HLA-DRB3*01:01 | 903 | ATYYLFDESGEFKLA | 0.04 | 0.23 | ||
| HLA-DQA1*01:02/DQB1*06:02 | 535 | AFASEAARVVRSIFS | 0.08 | −0.02 | ||
| HLA-DRB1*15:01 | 747 | AMPNMLRIMASLVLA | 0.01 | 0.09 | ||
| HLA-DRB3*02:02 | 2720 | AFVTNVNASSSEAFL | 0.01 | 0.15 | ||
| HLA-DRB1*11:01 | 865 | NEFYAYLRKHFSMMI | 0.02 | 0.22 | ||
| HLA-DQA1*05:01/DQB1*02:01 | 2390 | QMEIDFLELAMDEFI | 0.03 | 0.61 | ||
| HLA-DRB3*02:02 | 2755 | NYIFWRNTNPIQLSS | 0.04 | 0.92 | ||
| HLA-DQA1*05:01/DQB1*02:01 | 2393 | IDFLELAMDEFIERY | 0.05 | 0.25 | ||
| HLA-DPA1*02:01/DPB1*14:01 | 663 | QMNLKYAISAKNRAR | 0.07 | 1.5 |
Table 3. Analysis of MHC II epitope’s allergenicity and toxicity.
| S. no | Protein name | Start | Peptide | Allergenicity | Toxicity |
|---|---|---|---|---|---|
| 1 | ORF8 protein | 14 | QPYVVDDPCPIHFYS | Allergen | – |
| 10 | CTQHQPYVVDDPCPI | Non-allergenic | Non-toxic | ||
| 13 | HQPYVVDDPCPIHFY | Non-allergenic | Non-toxic | ||
| 12 | QHQPYVVDDPCPIHF | Allergen | – | ||
| 11 | TQHQPYVVDDPCPIH | Non-allergenic | Non-toxic | ||
| 2 | Surface glycoprotein | 98 | KTQSLLIVNNATNVV | Non-allergenic | Non-toxic |
| 100 | QSLLIVNNATNVVIK | Non-allergenic | Non-toxic | ||
| 101 | SLLIVNNATNVVIKV | Non-allergenic | Non-toxic | ||
| 99 | TQSLLIVNNATNVVI | Non-allergenic | Non-toxic | ||
| 100 | QSLLIVNNATNVVIK | Non-allergenic | Non-toxic | ||
| 498 | LSFELLHAPATVCGP | Non-allergenic | Non-toxic | ||
| 497 | VLSFELLHAPATVCG | Non-allergenic | Non-toxic | ||
| 496 | VVLSFELLHAPATVC | Non-allergenic | Non-toxic | ||
| 97 | SKTQSLLIVNNATNV | allergenic | – | ||
| 101 | SLLIVNNATNVVIKV | Non-allergenic | Non-toxic | ||
| 99 | TQSLLIVNNATNVVI | Non-allergenic | Non-toxic | ||
| 495 | VVVLSFELLHAPATV | Non-allergenic | Non-toxic | ||
| 3 | ORF1ab polyprotein | 20 | KSAFYILPSIISNEK | Non-allergenic | Non-toxic |
| 2165 | CTNYMPYFFTLLLQL | allergenic | – | ||
| 1801 | ESPFVMMSAPPAQYE | Non-allergenic | Non-toxic | ||
| 2390 | QMEIDFLELAMDEFI | Non-allergenic | Non-toxic | ||
| 2755 | NYIFWRNTNPIQLSS | Non-allergenic | Non-toxic | ||
| 663 | QMNLKYAISAKNRAR | Non-allergenic | Non-toxic |
B cell epitope prediction with antigenicity, no allergenicity and no toxicity
Using the IEDB Bepipred server, different epitopes from selected proteins like ORF8 protein (two epitopes), ORF7a protein (two epitopes), membrane glycoprotein (one epitope), envelope protein (one epitope), ORF3a protein (one epitope), surface glycoprotein (28 epitopes) and ORF1ab protein (98 epitopes) have been screened. With ABCpred server-based B cell epitope analysis, the epitope of ORF8 protein (nine epitopes), ORF7a protein (seven epitopes), membrane glycoprotein (one epitope), envelope protein (three epitopes), ORF3a protein (two epitopes), surface glycoprotein (50 epitopes) and ORF1ab protein (20 epitopes) was shortlisted. To filter out the common epitope from both servers, manual screening has been done to shortlist the common overlapped epitopes. This selects different epitopes from Orf8 protein (two epitopes), ORF7a protein (two epitopes), membrane glycoprotein (one epitope), envelope protein (one epitope), ORF3a protein (one epitope), surface glycoprotein (16 epitopes) and ORF1ab protein (eight epitopes). These epitopes were selected for the further experiment (Table 4). The screened epitopes were further analyzed for antigenicity, allergenicity and toxicity (Table 5). This resulted in shortlisting of ORF8 protein (one epitope), envelope protein (one epitope), ORF3a protein (one epitope), surface glycoprotein (nine epitopes) and ORF1ab protein (six epitopes) epitopes for further study.
Table 4. Comparative analysis of B cell epitope identified using IEDB and ABCpred server and antigenicity analysis.
| S. no | Protein name | Start | Epitope IEDB BepiPred | Antigenicity | ABCpred | Start | Antigenicity |
|---|---|---|---|---|---|---|---|
| 1 | ORF8 protein | 12 | QHQPYVVDDP | 0.4127 | PYVVDDPCPIHFYSKW | 15 | 0.56 |
| 49 | EAGSKSPI | 0.2081 | ELCVDEAGSKSPIQYI | 44 | −0.18 | ||
| 2 | ORF7a protein | 18 | EPCSSGTYEGNSPFHPLAD | 0.39 | SGTYEGNSPFHPLADN | 22 | 0.29 |
| 58 | HVYQLRARSVSPKLFIRQE | 0.59 | HVYQLRARSVSPKLFI | 58 | 0.43 | ||
| 3 | Membrane glycoprotein | 7 | TITVEELKK | 0.57 | DSNGTITVEELKKLLE | 3 | 0.07 |
| 4 | Envelope protein | 23 | YSRVKNLNSSRVP | 0.47 | YVYSRVKNLNSSRVPD | 21 | 0.54 |
| 5 | ORF3a protein | 15 | LKQGEIKDATPSDFVR | 0.81 | QGEIKDATPSDFVRAT | 17 | 0.91 |
| IGTVTLKQGEIKDATP | 10 | 1.22 | |||||
| 6 | Surface glycoprotein | 55 | VSGTNGTKRF | 0.53 | HRSYLTPGDSSSGWTA | 230 | 0.6 |
| 123 | DPFLGVYYHKNNKSWMESEFRVYSSA | 0.49 | TVEKGIYQTSNFRVQP | 292 | 0.67 | ||
| 234 | LTPGDSSSGWTA | 0.68 | GCLIGAEHVNNSYECD | 633 | 0.84 | ||
| 298 | YQTSNFRVQP | 1.18 | LQSYGFQPTNGVGYQP | 477 | 0.52 | ||
| 315 | PNITNLCPFGEVFNATRFASVYAWNRKRISNC | 0.47 | TEIYQAGSTPCNGVEG | 455 | −0.01 | ||
| 389 | GDEVRQIAPGQTGKIAD | 1.06 | KQIYKTPPIKDFGGFN | 771 | −0.22 | ||
| 441 | FRKSNLKPFERDISTEIYQAGSTPCNGVEGFNCYFPLQSYGFQPT | 0.39 | CGPKKSTNLVKNKCVN | 510 | 0.2 | ||
| 501 | ELLHAPATVCGPKKSTNLVKN | 0.0029 | FERDISTEIYQAGSTP | 449 | −0.29 | ||
| 619 | RVYSTGSNVFQ | −0.1 | SWMESEFRVYSSANNC | 136 | 0.17 | ||
| 641 | VNNSYECDIPI | 0.6124 | EVRQIAPGQTGKIADY | 391 | 1.38 | ||
| 657 | ASYQTQTNSPRRARSVASQ | 0.2556 | TPTWRVYSTGSNVFQT | 615 | 0.18 | ||
| 680 | YTMSLGAENSVAYSNN | 0.6434 | VIGIVNNTVYDPLQPE | 1114 | 0.71 | ||
| 771 | KQIYKTPPIKDFGGF | −0.3896 | SQSIIAYTMSLGAENS | 674 | 0.56 | ||
| 792 | PDPSKPSKR | 0.478 | VSGTNGTKRFDNPVLP | 55 | 0.51 | ||
| 1093 | NFYEPQIITTD | 0.36 | FPNITNLCPFGEVFNA | 314 | 0.6 | ||
| 1118 | VNNTVYDPLQPELDSFKEELDKYFKNHTSPDVDLGDISG | 0.13 | SQILPDPSKPSKRSFI | 788 | 0.26 | ||
| YQTQTNSPRRARSVAS | 659 | 0.192 | |||||
| 7 | Orf1ab polyprotein | 373 | CHNSEVGPEH | 1.14 | VVKIYCPACHNSEVGP | 365 | 0.76 |
| 763 | LQPLEQPTSEAVEAP | 0.05 | TLKGGAPTKVTFGDDT | 814 | 0.98 | ||
| 813 | FTLKGGAP | 0.88 | TSRYWEPEFYEAMYTP | 3996 | 0.4 | ||
| 1458 | NLEEAAR | −0.14 | AVTAYNGYLTSSSKTP | 1482 | 0.35 | ||
| 1482 | AVTAYNGYLTSSSKTPEE | 0.5 | LNLEEAARYMRSLKVP | 1457 | 0.33 | ||
| 2241 | FSSEIIGYKAI | 0.26 | SEAVEAPLVGTPVCIN | 771 | 0.74 | ||
| 3072 | GCSCDQLREPMLQSADAQS | 0.92 | CGMWKGYGCSCDQLRE | 3065 | 0.17 | ||
| 3993 | NDNTSRYWEP | 0.27 | SSEIIGYKAIDGGVTR | 2242 | 0.74 |
Table 5. Analysis of antigenic B-Cell epitope’s allergenicity and toxicity.
| S. no | Protein name | Start | Epitope IEDB bepipred | Allergenicity | Toxicity | ABCpred | Start | Allergenicity | Toxicity |
|---|---|---|---|---|---|---|---|---|---|
| 1 | ORF8 protein | 12 | QHQPYVVDDP | Allergenic | – | PYVVDDPCPIHFYSKW | 15 | Non-allergenic | |
| 2 | ORF7a protein | 58 | HVYQLRARSVSPKLFIRQE | Allergenic | – | HVYQLRARSVSPKLFI | 58 | Allergen | – |
| 3 | Membrane glycoprotein | 7 | TITVEELKK | Allergenic | – | ||||
| 4 | Envelope protein | 23 | YSRVKNLNSSRVP | Non-Allergenic | Non-toxic | YVYSRVKNLNSSRVPD | 21 | Allergen | – |
| 5 | ORF3a protein | 15 | LKQGEIKDATPSDFVR | Non-Allergenic | Non-toxic | QGEIKDATPSDFVRAT | 17 | Allergen | – |
| IGTVTLKQGEIKDATP | 10 | Allergen | – | ||||||
| 6 | Surface glycoprotein | 55 | VSGTNGTKRF | Non-Allergenic | Non-toxic | HRSYLTPGDSSSGWTA | 230 | Non-allergenic | Non-toxic |
| 123 | DPFLGVYYHKNNKSWMESEFRVYSSA | Non-Allergenic | Non-toxic | TVEKGIYQTSNFRVQP | 292 | Allergen | – | ||
| 234 | LTPGDSSSGWTA | Non-Allergenic | Non-toxic | GCLIGAEHVNNSYECD | 633 | Non-allergenic | Toxic | ||
| 298 | YQTSNFRVQP | Allergenic | – | LQSYGFQPTNGVGYQP | 477 | Non-allergenic | Non-toxic | ||
| 315 | PNITNLCPFGEVFNATRFASVYAWNRKRISNC | Allergenic | – | EVRQIAPGQTGKIADY | 391 | Non-allergenic | Non-toxic | ||
| 389 | GDEVRQIAPGQTGKIAD | Non-Allergenic | Non-toxic | TPTWRVYSTGSNVFQT | 615 | Non-allergenic | Non-toxic | ||
| 641 | VNNSYECDIPI | Non-Allergenic | Non-toxic | VIGIVNNTVYDPLQPE | 1114 | Non-allergenic | Non-toxic | ||
| 680 | YTMSLGAENSVAYSNN | Non-Allergenic | Non-toxic | SQSIIAYTMSLGAENS | 674 | Non-allergenic | Non-toxic | ||
| 792 | PDPSKPSKR | Non-Allergenic | Non-toxic | VSGTNGTKRFDNPVLP | 55 | Allergen | – | ||
| FPNITNLCPFGEVFNA | 314 | Allergen | – | ||||||
| 7 | Orf1ab polyprotein | 373 | CHNSEVGPEH | Allergenic | – | VVKIYCPACHNSEVGP | 365 | Non-allergenic | Non-toxic |
| 813 | FTLKGGAP | Non-Allergenic | Non-toxic | TLKGGAPTKVTFGDDT | 814 | Allergen | – | ||
| 1482 | AVTAYNGYLTSSSKTPEE | Non-Allergenic | Non-toxic | TSRYWEPEFYEAMYTP | 3996 | Allergen | – | ||
| 3072 | GCSCDQLREPMLQSADAQS | Non-Allergenic | Non-toxic | SEAVEAPLVGTPVCIN | 771 | Non-allergenic | Non-toxic | ||
| SSEIIGYKAIDGGVTR | 2242 | Non-allergenic | Non-toxic |
Cross-reactivity, IFN gamma induction analysis of MHC I, II and B cell epitopes
All finalized MHC I, II and B cell epitopes were used for comparative analysis (Table 6) to remove the overlapping sequences. In addition to that, cross-reactivity of selected epitopes with humans has been predicated for analyzing similarity between virus protein epitopes and host cells peptide, which eliminates autoimmune reactivity. BLAST result of all selected epitopes against the human proteome showed that no epitopes were found to have cross-reactivity reactions in the host cell. In addition to this, it was seen that in virus-human cell immune response activity, IFN gamma plays an important role. High expression of IFN gamma leads to virus clearance from human cells. IFNepitope server positive score showed the capacity of the epitope to induce the IFN secretion via T cells (TC), which was listed in Table 6. All the selected epitopes were used to design the different vaccine constructs.
Table 6. Comparative analysis of Final MHC I, MHC II and B cell epitopes.
| S. no | Protein name | Start | B cell epitopes (IFNscore) | Cross-reactivity with human | START | MHC I epitopes (IFNscore) | Cross-reactivity with human | Start | MHC II Epitopes (IFNscore) | Cross-reactivity with human |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ORF8 protein | 15 | PYVVDDPCPIHFYSKW(1) | NO | 10 | CTQHQPYVVDDPCPI(1) | NO | |||
| 2 | Envelope protein | 23 | YSRVKNLNSSRVP(−0.49) | NO | ||||||
| 3 | ORF3a protein | 15 | LKQGEIKDATPSDFVR(0.62) | NO | 4 | MRIFTIGTV(−0.43) | NO | |||
| 4 | Surface glycoprotein | 55 | VSGTNGTKRF(−0.75) | NO | 242 | WTAGAAAYY(0.57) | NO | 98 | KTQSLLIVNNATNVV(−0.37) | NO |
| 123 | DPFLGVYYHKNNKSWMESEFRVYSSA(3.7) | NO | 498 | LSFELLHAPATVCGP(−0.51) | NO | |||||
| 234 | LTPGDSSSGWTA(−0.14) | NO | – | – | – | – | – | – | ||
| 641 | VNNSYECDIPI(1) | NO | – | – | – | – | – | – | ||
| 680 | YTMSLGAENSVAYSNN(−0.04) | NO | – | – | – | – | – | |||
| 792 | PDPSKPSKR(0.12) | NO | – | – | – | – | – | – | ||
| 477 | LQSYGFQPTNGVGYQP(14) | NO | ||||||||
| 391 | EVRQIAPGQTGKIADY(1) | NO | ||||||||
| 1114 | VIGIVNNTVYDPLQPE(12) | NO | ||||||||
| 5 | Orf1ab polyprotein | 365 | VVKIYCPACHNSEVGP(2) | NO | 3841 | LSDDAVVCF(−0.30) | NO | 20 | KSAFYILPSIISNEK(0.33) | NO |
| 771 | SEAVEAPLVGTPVCIN(2) | NO | 2413 | VVTTFDSEY(−0.51) | NO | 1801 | ESPFVMMSAPPAQYE(−0.21) | NO | ||
| 2242 | SSEIIGYKAIDGGVTR(2) | NO | 295 | FMGRIRSVY(−0.25) | NO | 2390 | QMEIDFLELAMDEFI(0.31) | NO | ||
| 813 | FTLKGGAP(−0.22) | NO | 4615 | LQAENVTGL(−0.21) | NO | 2755 | NYIFWRNTNPIQLSS(−0.27) | NO | ||
| 1482 | AVTAYNGYLTSSSKTPEE(−0.04) | NO | 2166 | NYMPYFFTL(−0.58) | NO | 663 | QMNLKYAISAKNRAR(1) | NO | ||
| 3072 | GCSCDQLREPMLQSADAQS(0.41) | NO | 5406 | FELEDFIPM(−0.28) | NO |
Analysis of physicochemical properties of selected vaccine construct VTC3
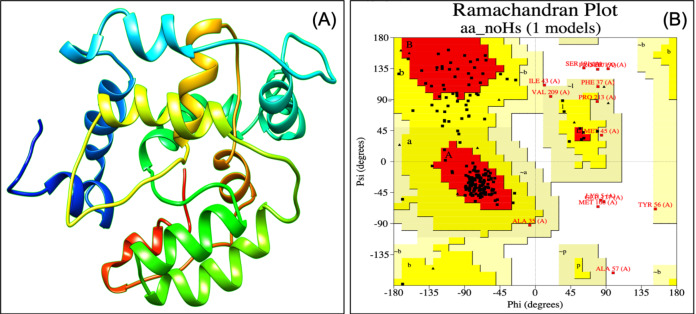
Designed vaccine constructs VTC1, VTC2 and VTC3 (Table 7), showed antigenicity with no allergenicity and no toxicity. The physiochemical analysis of highly antigenic vaccine construct VTC3 showed that it has 227 amino acids, a molecular weight of 24 kDa, and an instability index of 23.36 that represent the protein’s stable nature. VTC3 construct contains the aliphatic and GRAVY index value of 68.72 and −0.455, respectively. PESIPRED secondary structure analysis of vaccine construct VTC3 showed the 62.56% alpha helicity, 7.05% extended strands, 5.73% beta-turn with 24.67% random coils. VTC3 tertiary structure has been modeled, refine, and validated by Ramachandran plot that showed 97% residue in the favored region (Fig. 2). The model has a Z-score of −3.35 and QMEAN of −10.75 (Fig. S1). To validate Phyre 2 model, the ab initio model was generated using RaptorX. Both models are similar, and the Ramachandran plot of the RaptoX model showed 99% residue in the favored region. This confirms that phyre2 model is valid hence used for further study.
Table 7. Sequence, Antigenicity, and Allergenicity of designed multi-epitope Vaccine constructs.
| S. no | Name | Vaccine construct | Antigenicity | Allergenicity |
|---|---|---|---|---|
| 1 | VTC1 | EAAAKCTQHQPYVVDDPCPIHEYGAEALERAGYSRVKNLNSSRVPGGGSMRIFTIGTVHEYGAEALERAGKTQSLLIVNNATNVVGGGSLKQGEIKDATPSDFVRHEYGAEALERAGWTAGAAAYYGGGSLSFELLHAPATVCGPHEYGAEALERAGVSGTNGTKRFGGGSLSDDAVVCFHEYGAEALERAGKSAFYILPSIISNEKGGGSVVKIYCPACHNSEVGPEAAAK | 0.51 | Non-allergenic |
| 2 | VTC2 | EAAAKCTQHQPYVVDDPCPIHEYGAEALERAGYSRVKNLNSSRVPEAAAKLKQGEIKDATPSDFVRHEYGAEALERAGMRIFTIGTVEAAAKKTQSLLIVNNATNVVHEYGAEALERAGDPFLGVYYHKNNKSWMESEFRVYSSAEAAAKWTAGAAAYYHEYGAEALERAGPDPSKPSKREAAAKNYIFWRNTNPIQLSSHEYGAEALERAG SSEIIGYKAIDGGVTREAAAK LQAENVTGL | 0.47 | Non-allergenic |
| 3 | VTC3 | EAAAKGCSCDQLREPMLQSADAQSHEYGAEALERAGFELEDFIPMEAAAKQMNLKYAISAKNRARHEYGAEALERAGEVRQIAPGQTGKIADYEAAAKLSFELLHAPATVCGPHEYGAEALERAGWTAGAAAYYEAAAKLKQGEIKDATPSDFVRHEYGAEALERAGMRIFTIGTVEAAAKYSRVKNLNSSRVPHEYGAEALERAG PYVVDDPCPIHFYSKWEAAAK | 0.61 | Non-allergenic |
Figure 2. Tertiary structure of modeled VTC3 construct (A) and Ramachandran plot of the modeled proteins (B).
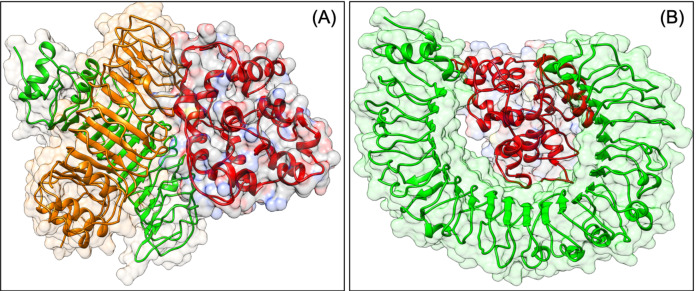
Molecular docking analysis confirms the interaction of VTC3 with TLR and HLA
To be effective against SARS-CoV2, a vaccine should have the capacity to activate the immune response of the human host. The virus can subvert the host protein function, which plays a role in host cell invasion or virus persistence. In a previous study, it has been shown that TLRs have an extensive role in pathogen persistence and clearance. Hence, docking analysis was performed, which showed that our construct VTC3 interacts with TLR1/2 complex, TLR1, TLR3, TLR4, TLR6 but has the highest binding affinity with TLR1/2 complex, followed by TLR4 and TLR6 (Table 8). The docked poses are shown in Fig. 3. As mentioned in the introduction, the interaction of VTC3 with TLR1/2 is important for the immune response for viral infection; hence docking results further confirm it. In addition to that, molecular docking of VTC3 with different HLAs (MHCs) has been performed, which was important to induce MHC-I and MHC-II to activate the immune response in the host cell. Best docked TLRs and HLA alleles molecules were further confirmed by the HDOCK server (Fig. S2). The binding affinity and dissociation constant of VTC3 were favorable with HLA, TLR1/2 and TLR4 (Table S1).
Table 8. Molecular docking of vaccine construct with different TLRs and HLA alleles.
| S. no. | Receptor | Receptor PDB | Global Energy |
|---|---|---|---|
| 1 | TLR4 | 2Z62 | −1.64 |
| 3UL8 | 2.16 | ||
| 2Z63 | −28.76 | ||
| 3UL9 | −1.43 | ||
| 2Z65 | −18.55 | ||
| 2Z66 | −23.62 | ||
| 3FXI | −6.93 | ||
| 3ULA | −1.75 | ||
| 4G8A | −2.65 | ||
| 5NAM | −6.18 | ||
| 2Z64 | 1.23 | ||
| 2 | TLR1-2 hetero dimer | 2Z81 | −0.88 |
| 2Z80 | −31.20 | ||
| 2Z82 | 0.22 | ||
| 2Z7X | 3.75 | ||
| 3 | TLR1 | 6NIH | −1.95 |
| 1FYV | −6.49 | ||
| 4 | TLR2 | 6NIG | 10.81 |
| 5 | TLR6 | 3A79 | −13.05 |
| 6 | TLR3 | 2A0Z | −8.85 |
| 1ZIW | 6.11 | ||
| 7 | HLA Alleles | 5IM7 | −24.66 |
| 1XR8 | −1.95 | ||
| 1SYS | −17 | ||
| 1A6A | −5.59 | ||
| 1A1M | −3.24 | ||
| 1BX2 | 6.09 | ||
| 1H15 | −9.32 | ||
| 1ZSD | 5.51 | ||
| 3C5J | 5.12 | ||
| 4O2E | 9.68 |
Figure 3. Docked pose of VTC3-TLR1/2 complex (A) and VTC3-TLR4 complex (B).
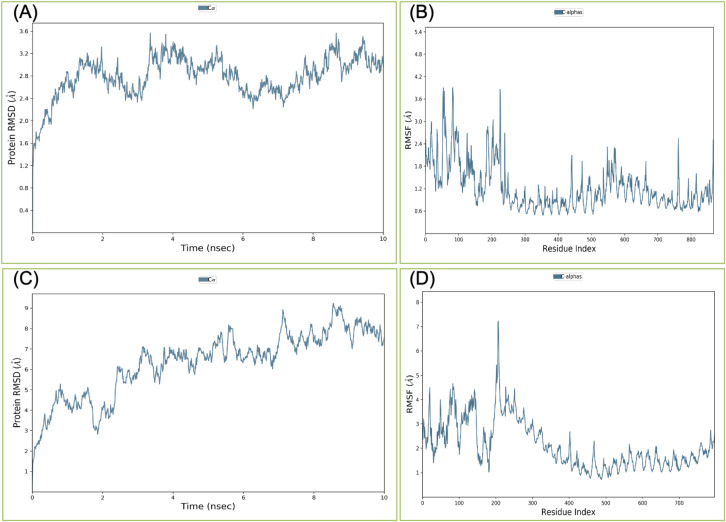
MDS analysis confirms the strong interaction of VTC3 with TLR1/2 heterodimer and HLA
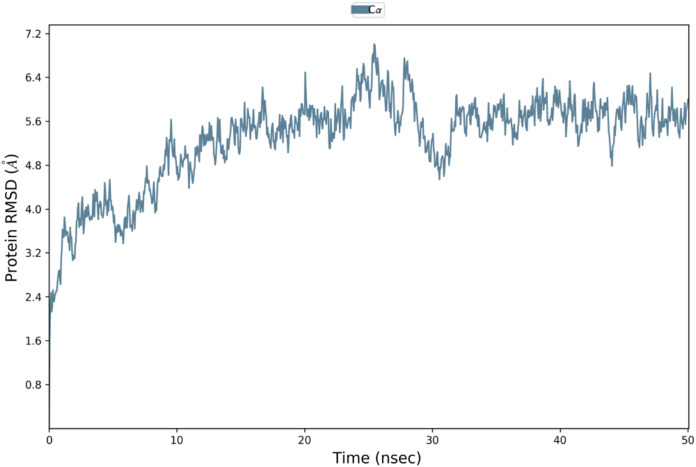
Binding affinity from the PatchDock result showed that VTC3 has better interaction with the TLR1/2 complex than TLR4, while Binding affinity from HDOCK result showed TLR4 have better interaction than TLR1/2; hence both complexes were analyzed by MDS analysis. MDS was performed till 10 ns for both the complex, and results were analyzed for RMSD, RMSF, etc. RMSD calculation showed that the VTC3-TLR1/2 complex is stable throughout simulation with RMSD of around 3 Å (Fig. 4A), and the VTC3-TLR4 was found to be relativity less stable with RMSD of 7 Å (Fig. 4C). Similarly, the RMSF analysis showed that the VTC3-TLR1/2 complex has less fluctuation with RMSF of around 3 Å (Fig. 4B) while the VTC3-TLR4 complex showed that the vaccine has an RMSF of 7 Å (Fig. 4D). MDS result suggests that multiepitope vaccine construct VTC3 showed a stronger and stable interaction with TLR1/2 complex compared to TLR4 (Fig. 4); this indicated that this vaccine construct induces an immune response suitable for clearance of SARS CoV2. For a vaccine construct, it is also important to interact with HLA. MDS analysis of the VTC3-HLA complex was investigated till 50 ns, and the result was analyzed for RMSD and RMSF. RMSD calculation showed that the VTC3-HLA complex is stable throughout simulation with RMSD of around 5 Å at 50 ns (Fig. 5), and RMSF analysis showed that the VTC3-HLA complex has some fluctuation at the terminal. Both the data suggest the interaction between VTC3 and HLA.
Figure 4. Root-mean-square deviation and Root mean square fluctuations during molecular dynamics simulation analysis of VTC3-TLR1/2 complex (A and B) and VTC3-TLR4 complex (C and D).
Result shows a stable interaction of VTC3 with TLR1/2 complex.
Figure 5. Root-mean-square deviation during molecular dynamics simulation analysis of VTC3-HLA complex. MDS was performed till 50 ns.
Strong binding affinity and favorable dissociation constant between VTC3 with HLA and TLRs during MDS analysis
To further validate interaction, binding affinity and dissociation constant were calculated for MDS trajectories and found that VTC3 has a binding affinity of −13.1 kcal/mol at 45 ns simulation time and dissociation constant of 2.4 × 10−10 M with HLA at 45 ns. Similarity, binding affinity and dissociation constant of VTC3 was found to be favorable with TLR1/2 (−11.5 kcal/mol and 3.8 × 10−9) and TLR4 (−9.1 kcal/mol and 2.1 × 10−7) at 10 ns simulation time. This showed good interaction between VTC3 with HLA and TLR1/2. The binding affinity and dissociation constant at different simulation times (Table S2) and interacting residues of the VTC3-HLA complex (Table S3) are shown in Supplemental Data.
Multiepitope vaccine VTC3 does not have significant similarity with the human gut microbiome
NCBI blast of VTC3 construct against the proteome of 226 gut flora showed no significant similarity (Table S4) between amino acid sequence of the multiepitope vaccine construct VTC3 and amino acid sequence of the human gut microbiome that further minimize the cross-reactivity in the host cell.
Mutational profiling of multiepitope vaccine VTC3 showed less mutation sensitivity
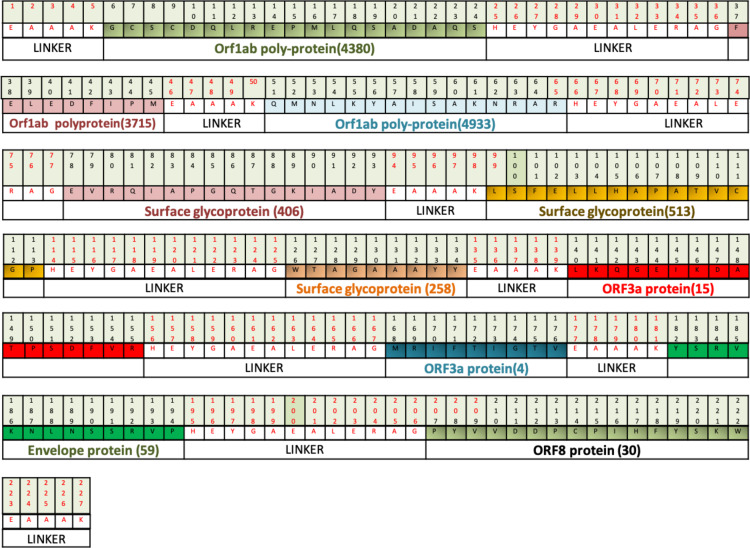
Mutational sensitivity profiling of the VTC3 vaccine construct (Fig. 6) showed that only a few epitope residues of VTC3 have ΔΔGpred > 0, suggesting that vaccine construct VTC3 has less mutation sensitivity, which enhances the possibility of vaccine constructs to be effective. Similarly, analysis of vaccine construct VTC3 in the CoV-GLUE database showed less mutation hot spots in our vaccine construct (Table S5). This database contains all replacements, insertions and deletions, which have been observed in the GISAID hCoV-19 sequence sampled from the pandemic. Interacting residues of vaccine construct VTC3 with the HLA, TLR1/2 complex, TLR4 has been identified. The result showed that most of the residues of VTC3 with the mutation do not involve the physical interaction with these proteins. These results further support the efficacy of the vaccine construct.
Figure 6. Diagrammatic presentation of proposed combinational chimeric multi-epitope vaccine VTC3 shows different epitopes and linkers in the vaccine.
Immune simulation confirms the strong immune response against the VTC3, as well as virus protection
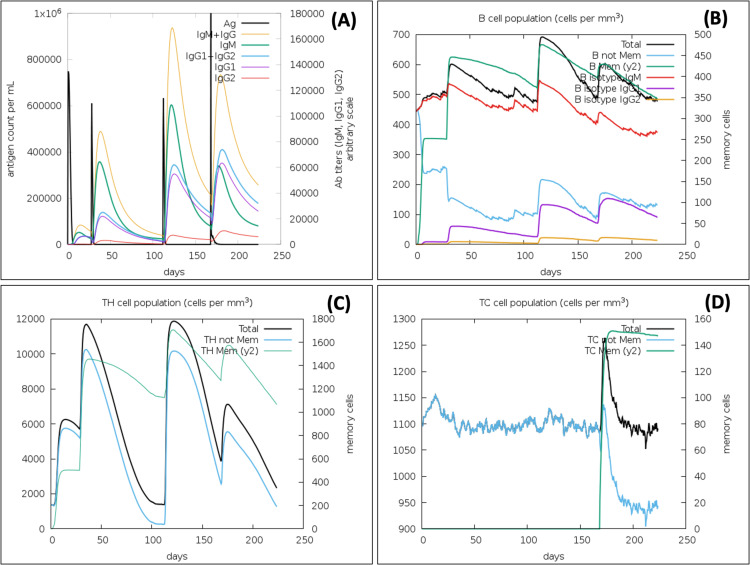
In the presence of antigen, the C-ImmSim model illustrates the host immune responses at both the humoral and the cellular level. The immune response against the VTC3 vaccine showed an appropriate immune response and virus antigen elimination (Fig. 7). Injection of the vaccine VTC3 without LPS shows elevated IgM level after the first injection. Its concentration further increases along with IgG1 after subsequent injection of VTC3, showing secondary and tertiary immune response. The secondary and tertiary response eliminates the antigen on a shorter timescale. It is also observed that when the virus was injected at 24 weeks (at 168 days), it is eliminated at the shortest timescale that showed the presence of memory cells to react with virus antigen. It was also observed that after virus injection, IgG1 titer is more than IgM titer (Fig. 7A). Similarly, B-cell population increases with subsequent injections of VTC3 that show a typical immune response. The memory B cell also increases after second injection and remains almost similar (Fig. 7B). The population of Th cells increases after injection of VTC3 and Th memory increase after second injection and remains almost same afterward (Fig. 7C). Among the Th cells, the population of Th1 is more dominant. After injection of VTC3, the cytotoxic TC population increases and remain constants after first injections with a TC memory (a.u. 900). It was observed that TC memory was further enhanced after injection of the virus at 168 days. It was also observed that VTC3 injection increases the IL-12 level and IFN-gamma showed a potent innate and adaptive immune response. The population of macrophages also increases after the injection of VTC3. Other cells like the dendritic cell, natural killer cells and epithelial cell population, plasma B lymphocyte population also support immune reaction. In brief, the result explains the development of strong immune memory with subsequent virus eliminating potential.
Figure 7. Immune Simulation of immunization experiment.
Injection of Vaccine VTC3 without LPS was given at 1st Day, 28th Days (4 Weeks), 112 Days (16 Weeks) and injection of the virus after 168 Days (24th week) after initial injection. (A) Antigen counts and immunoglobulins response in isotypes; (B) B-cell population observed with the administration of VTC3 and virus; (C) T helper (TH) cells population after injection of VTC3 and virus; (D) cytotoxic-T (TC) cells population after injection of VTC3 and virus. The result shows the virus antigen was eliminated at a shorter timescale due to memory cells’ presence ready to react against the epitopes.
Codon adaptation and in silico cloning
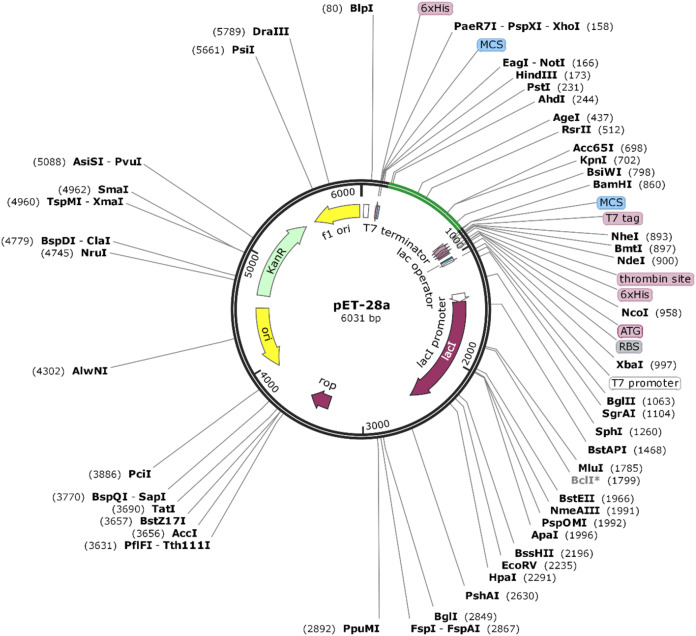
The Java Codon Adaptation tool converted the protein sequence of VTC3 into cDNA sequence. This cDNA sequence contains 52% GC content and CAI value of 1.0. This high CAI value shows a high expression of the vaccine gene in the host cell. The final vaccine construct (VTC3) was cloned into a pET28a vector between the HindIII and BamHI (Fig. 8).
Figure 8. In silico cloning of the SARS-CoV-2 vaccine in the vector, pET28a (+).
Green areas represent the COVID-19 vaccine, while the black areas represent the expression vector, pET28a (+).
Discussion
In the present study, a multiepitope vaccine constructs VTC3 for SARS CoV2 have been proposed with the help of reverse vaccinology that compiles the outer surface-exposed epitopes of the structural and non-structural proteins of this virus. There are different therapeutic strategies, and ongoing research has been tried to control SARS CoV2 (Felsenstein et al., 2020; Tiwari, 2020b, 2020a). Although different groups have tried to design a vaccine against SARS-CoV2, the present study reports a comprehensive approach to design a vaccine construct that taking care of all possible design shortcomings such as absence of surface epitopes, inappropriate TLRs interactions, less population coverage, interaction with the human microbiome, presence of cross-reactivity with human proteins, epitope form mutation hot spots and absence of immune response, etc. SARS CoV2 structural proteins like surface glycoprotein, membrane and envelope protein have a significant contribution in surface adherence and internalization, while non-structural protein is virulence-associated factors that cause immune-pathogenesis. SARS CoV2 host cell internalization is facilitated by surface glycoprotein (spike protein) interaction with the ACE2 receptor. Targeting the outer exposed peptides of both structural and non-structural protein as a vaccine target would be a promising approach to induce both humoral and cellular immune response that recognizes the virus and killed it. These proteins were shortlisted by antigenicity score and trans-membrane helicity. The shortlisted proteins were used to identify surface-exposed peptides. Surface exposed peptides of these proteins were further analyzed to determine MHC I, II and B cell-mediated epitopes. The selected epitopes were shortlisted by their antigenicity, allergenicity, toxicity, cross-reactivity, immunogenicity and IFN gamma secretion scores. Cross-reactivity of selected epitopes of the virus with the human’s protein has also predicted that eliminates autoimmune reactivity for the vaccine construct. These epitopes were used to design different vaccine constructs (VTC1, VTC2 and VTC3) with the help of linkers. Antigenicity, allergenicity and physiochemical analysis of vaccine constructs were further determined to enhance the peptide potency. For designing the reliable vaccine, a mutational hot spot site has been analyzed, and found that epitopes belong to the non-mutation site of the protein. To minimize similarity with human proteome and gut microbiome, BLASTP analysis has been done that showed no similarity of VTC3 construct with host cell proteins.
The finalized construct VTC3 should have the potential to interact with pathogen-associated molecular patterns, activates the host’s innate immune system, and consonance of the adaptive immunity via TLRs (TLR1, 2, 3, 4, 6, 7, 8 and 9). It has been observed that the TLRs mediated virus interaction not-only combat virus virulence and infection but also sometimes initiate the host system to overturn downstream signals for the benefit (replication and survival) of the virus-cell. During the viral infection, membrane-associated proteins interaction with TLR2 and TLR4 plays a nuanced role in exceeding inflammatory responses via adhesion and invasion that vitiate the host cell (Olejnik, Hume & Muhlberger, 2018). Simultaneously with TLRs, HLA alleles have a significant contribution to the activation of the robust immune response. The strong HLA-epitope interaction would maintain the signaling cascades to activate the immune response for the viral infection clearance. To prove the modeled VTC3 construct, we have docked it with different HLAs and TLRs. Presently, the focus lies on the development of vaccines against viruses that can activate the innate immune system via TLR1/2 (Carty & Bowie, 2010; Dowling & Mansell, 2016; Jensen et al., 2018) to overcome the subvert effect of the virus on TLR mediated signaling. It is reported that the CD8+ T cell-mediated HLA allele (HLA-B*5801, PDB ID 5IM7) unique interaction with immune dominant peptide contributes as a potential to control and prevent viral infection (Li et al., 2016). The interaction of VTC3 was investigated with molecular docking and MDS, and the result is in agreement that the VTC3 construct has the highest affinity with allele HLA-B*5801. It might also create a ray of hope for the potential creation of vaccines or convalescent serum antibodies against SARS CoV2. Vaccine behaviors in human is a significant concern hence immune simulation was performed that shows strong immune response after injection of VTC3, and virus elimination. The designed multiepitope vaccine construct VTC3 should be tested experimentally for therapeutic potency in future studies. Experimental validation is necessary to demonstrate the potency of the designed vaccine in future studies.
Conclusion
The high mortality and morbidity rate of COVID 19 is unprecedented due to the unavailability of vaccination; hence effective treatment strategies (inhibitor, drugs, or vaccine), rapid development, trials and production are needed immediately for this global pandemic disease. To reach out the success, it is necessary to evaluate all possible vaccine candidates to find out the one viable outcome. To increase the chance of success, WHO initiated vaccine solidarity trials to test all vaccine candidates until they fail. In the present study, an attempt was made to design a multi-epitope vaccine against SARS CoV2 that targets its exposed peptides of structural and non-structural proteins. Immunogenicity, allergenicity, toxicity and potent IFN-gamma inducer scores have also been analyzed to narrow down efficient epitopes further. Peptide matching with the human proteome showed no indication of possible cross-reactivity. However, current reverse immuno-informatics approaches were executed to target surface-exposed proteins for enhancing effective host innate with humoral and cellular immune responses. The present study concludes the design of VTC3 as a multiepitope vaccine against SARS CoV2.
Supplemental Information
Acknowledgments
VT would like to thank the Central University of Rajasthan for providing the Schrodinger suite.
Funding Statement
The authors received no funding for this work.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Vandana Solanki performed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Monalisa Tiwari performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Vishvanath Tiwari conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
Raw data are available as a Supplemental File.
References
- Ahmad et al. (2019).Ahmad B, Ashfaq UA, Rahman MU, Masoud MS, Yousaf MZ. Conserved B and T cell epitopes prediction of ebola virus glycoprotein for vaccine development: an immuno-informatics approach. Microbial Pathogenesis. 2019;132(4):243–253. doi: 10.1016/j.micpath.2019.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alamri, Tahir ul Qamar & Alqahtani (2020).Alamri M, Tahir ul Qamar M, Alqahtani S. Pharmacoinformatics and molecular dynamic simulation studies reveal potential inhibitors of SARS-CoV-2 main protease 3CLpro. Journal of Biomolecular Structure and Dynamics. 2020:1–13. doi: 10.1080/07391102.2020.1782768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benvenuto et al. (2020).Benvenuto D, Giovanetti M, Ciccozzi A, Spoto S, Angeletti S. The 2019-new coronavirus epidemic: evidence for virus evolution. Journal of Medical Virology. 2020;92(4):455–459. doi: 10.1002/jmv.25688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennick et al. (2017).Brennick CA, George MM, Corwin WL, Srivastava PK, Ebrahimi-Nik H. Neoepitopes as cancer immunotherapy targets: key challenges and opportunities. Immunotherapy. 2017;9(4):361–371. doi: 10.2217/imt-2016-0146. [DOI] [PubMed] [Google Scholar]
- Buchan & Jones (2019).Buchan DWA, Jones DT. The PSIPRED protein analysis workbench: 20 years on. Nucleic Acids Research. 2019;47(W1):W402–W407. doi: 10.1093/nar/gkz297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calis et al. (2013).Calis JJ, Maybeno M, Greenbaum JA, Weiskopf D, De Silva AD, Sette A, Kesmir C, Peters B. Properties of MHC class I presented peptides that enhance immunogenicity. PLOS Computational Biology. 2013;9(10):e1003266. doi: 10.1371/journal.pcbi.1003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty & Bowie (2010).Carty M, Bowie AG. Recent insights into the role of Toll-like receptors in viral infection. Clinical & Experimental Immunology. 2010;161(3):397–406. doi: 10.1111/j.1365-2249.2010.04196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan et al. (2019).Chauhan V, Rungta T, Goyal K, Singh MP. Designing a multi-epitope based vaccine to combat Kaposi Sarcoma utilizing immunoinformatics approach. Scientific Reports. 2019;9(1):2517. doi: 10.1038/s41598-019-39299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2013).Chen C, Li Z, Huang H, Suzek BE, Wu CH, UniProt Consortium A fast peptide match service for UniProt knowledgebase. Bioinformatics. 2013;29(21):2808–2809. doi: 10.1093/bioinformatics/btt484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Liu & Guo (2020).Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. Journal of Medical Virology. 2020;92(4):418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gregorio & Rappuoli (2012).De Gregorio E, Rappuoli R. Vaccines for the future: learning from human immunology. Microbial Biotechnology. 2012;5(2):149–155. doi: 10.1111/j.1751-7915.2011.00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wilde et al. (2018).De Wilde AH, Snijder EJ, Kikkert M, Van Hemert MJ. Host factors in coronavirus replication. Current Topics in Microbiology and Immunology. 2018;419(7):1–42. doi: 10.1007/82_2017_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov et al. (2013).Dimitrov I, Naneva L, Doytchinova I, Bangov I. AllergenFP: allergenicity prediction by descriptor fingerprints. Bioinformatics. 2013;30(6):846–851. doi: 10.1093/bioinformatics/btt619. [DOI] [PubMed] [Google Scholar]
- Dowling & Mansell (2016).Dowling JK, Mansell A. Toll-like receptors: the swiss army knife of immunity and vaccine development. Clinical & Translational Immunology. 2016;5(5):e85. doi: 10.1038/cti.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doytchinova & Flower (2007).Doytchinova IA, Flower DR. VaxiJen: a server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinformatics. 2007;8(1):4. doi: 10.1186/1471-2105-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein et al. (2020).Felsenstein S, Herbert JA, McNamara PS, Hedrich CM. COVID-19: immunology and treatment options. Clinical Immunology. 2020;215(1):108448. doi: 10.1016/j.clim.2020.108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasteiger et al. (2003).Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Research. 2003;31(13):3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya et al. (2020).Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, Haagmans BL, Lauber C, Leontovich AM, Neuman BW, Penzar D, Perlman S, Poon LLM, Samborskiy D, Sidorov IA, Sola I, Ziebuhr J. Severe acute respiratory syndrome-related coronavirus: the species and its viruses—a statement of the coronavirus study group. bioRxiv. 2020 doi: 10.1101/2020.02.07.937862. [DOI] [Google Scholar]
- Greenbaum et al. (2011).Greenbaum J, Sidney J, Chung J, Brander C, Peters B, Sette A. Functional classification of class II human leukocyte antigen (HLA) molecules reveals seven different supertypes and a surprising degree of repertoire sharing across supertypes. Immunogenetics. 2011;63(6):325–335. doi: 10.1007/s00251-011-0513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta et al. (2013).Gupta S, Kapoor P, Chaudhary K, Gautam A, Kumar R, Raghava GP. In silico approach for predicting toxicity of peptides and proteins. PLOS ONE. 2013;8(9):e73957. doi: 10.1371/journal.pone.0073957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang et al. (2020).Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen & Andreatta (2018).Jensen KK, Andreatta M. Improved methods for predicting peptide binding affinity to MHC class II molecules. Immunology. 2018;154(3):394–406. doi: 10.1111/imm.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen et al. (2018).Jensen KK, Andreatta M, Marcatili P, Buus S, Greenbaum JA, Yan Z, Sette A, Peters B, Nielsen M. Improved methods for predicting peptide binding affinity to MHC class II molecules. Immunology. 2018;154(3):394–406. doi: 10.1111/imm.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen et al. (2017).Jespersen MC, Peters B, Nielsen M, Marcatili P. BepiPred-2.0: improving sequence-based B-cell epitope prediction using conformational epitopes. Nucleic acids research. 2017;45(W1):W24–W29. doi: 10.1093/nar/gkx346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiayuan et al. (2020).Jiayuan C, Jinsong S, Tungon Y, Chang L, Xin L, Qiang Z, Jishou R, Gao S. Bioinformatics analysis of the 2019 novel coronavirus genome. Chinese Journal of Bioinformatics. 2020;18:96–102. doi: 10.12113/202001007. [DOI] [Google Scholar]
- Kelley et al. (2015).Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJE. The Phyre2 web portal for protein modeling, prediction and analysis. Nature Protocols. 2015;10(6):845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan et al. (2018).Khan A, Junaid M, Kaushik AC, Ali A, Ali SS, Mehmood A, Wei DQ. Computational identification, characterization and validation of potential antigenic peptide vaccines from hrHPVs E6 proteins using immunoinformatics and computational systems biology approaches. PLOS ONE. 2018;13(5):e0196484. doi: 10.1371/journal.pone.0196484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh et al. (2001).Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. Journal of Molecular Biology. 2001;305(3):567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- Laimer et al. (2016).Laimer J, Hiebl-Flach J, Lengauer D, Lackner P. MAESTROweb: a web server for structure-based protein stability prediction. Bioinformatics. 2016;32(9):1414–1416. doi: 10.1093/bioinformatics/btv769. [DOI] [PubMed] [Google Scholar]
- Li & De Clercq (2020).Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nature Reviews Drug Discovery. 2020;19(3):149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- Li et al. (2016).Li X, Lamothe PA, Ng R, Xu S, Teng M, Walker BD, Wang J-H. Crystal structure of HLA-B*5801, a protective HLA allele for HIV-1 infection. Protein & Cell. 2016;7(10):761–765. doi: 10.1007/s13238-016-0309-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu & Meng (2017).Lu C, Meng S. A novel multi-epitope vaccine from MMSA-1 and DKK1 for multiple myeloma immunotherapy. British Journal of Haematology. 2017;178(3):413–426. doi: 10.1111/bjh.14686. [DOI] [PubMed] [Google Scholar]
- Mirza et al. (2016).Mirza MU, Rafique S, Ali A, Munir M, Ikram N, Manan A, Salo-Ahen OM, Idrees M. Towards peptide vaccines against Zika virus: immunoinformatics combined with molecular dynamics simulations to predict antigenic epitopes of Zika viral proteins. Scientific Reports. 2016;6(1):37313. doi: 10.1038/srep37313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nain et al. (2020).Nain Z, Karim MM, Sen MK, Adhikari UK. Structural basis and designing of peptide vaccine using PE-PGRS family protein of Mycobacterium ulcerans—an integrated vaccinomics approach. Molecular Immunology. 2020;120(7):146–163. doi: 10.1016/j.molimm.2020.02.009. [DOI] [PubMed] [Google Scholar]
- Olejnik, Hume & Muhlberger (2018).Olejnik J, Hume AJ, Muhlberger E. Toll-like receptor 4 in acute viral infection: too much of a good thing. PLOS Pathogens. 2018;14(12):e1007390. doi: 10.1371/journal.ppat.1007390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omasits et al. (2014).Omasits U, Ahrens CH, Muller S, Wollscheid B. Protter: interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics. 2014;30(6):884–886. doi: 10.1093/bioinformatics/btt607. [DOI] [PubMed] [Google Scholar]
- Patronov & Doytchinova (2013).Patronov A, Doytchinova I. T-cell epitope vaccine design by immunoinformatics. Open Biology. 2013;3(1):120139. doi: 10.1098/rsob.120139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman (2020).Perlman S. Another decade, another coronavirus. New England Journal of Medicine. 2020;382(8):760–762. doi: 10.1056/NEJMe2001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson et al. (2009).Peterson J, Garges S, Giovanni M, McInnes P, Wang L, Schloss JA, Bonazzi V, McEwen JE, Wetterstrand KA, Deal C. The NIH human microbiome project. Genome Research. 2009;19(12):2317–2323. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell, McCluskey & Rossjohn (2007).Purcell AW, McCluskey J, Rossjohn J. More than one reason to rethink the use of peptides in vaccine design. Nature Reviews Drug Discovery. 2007;6(5):404–414. doi: 10.1038/nrd2224. [DOI] [PubMed] [Google Scholar]
- Ramos et al. (2018).Ramos PIP, Do Porto DF, Lanzarotti E, Sosa EJ, Burguener G, Pardo AM, Klein CC, Sagot M-F, De Vasconcelos ATR, Gales AC, Marti M, Turjanski AG, Nicolás MF. An integrative, multi-omics approach towards the prioritization of Klebsiella pneumoniae drug targets. Scientific Reports. 2018;8(1):10755. doi: 10.1038/s41598-018-28916-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapin et al. (2010).Rapin N, Lund O, Bernaschi M, Castiglione F. Computational immunology meets bioinformatics: the use of prediction tools for molecular binding in the simulation of the immune system. PLOS ONE. 2010;5(4):e9862. doi: 10.1371/journal.pone.0009862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadi, Karkhah & Nouri (2017).Saadi M, Karkhah A, Nouri HR. Development of a multi-epitope peptide vaccine inducing robust T cell responses against brucellosis using immunoinformatics based approaches. Infection, Genetics and Evolution. 2017;51(43–51):227–234. doi: 10.1016/j.meegid.2017.04.009. [DOI] [PubMed] [Google Scholar]
- Saha & Raghava (2006).Saha S, Raghava GP. Prediction of continuous B-cell epitopes in an antigen using recurrent neural network. Proteins: Structure, Function, and Bioinformatics. 2006;65(1):40–48. doi: 10.1002/prot.21078. [DOI] [PubMed] [Google Scholar]
- Schneidman-Duhovny et al. (2005).Schneidman-Duhovny D, Inbar Y, Nussinov R, Wolfson HJ. PatchDock and SymmDock: servers for rigid and symmetric docking. Nucleic Acids Research. 2005;33(Web Server):W363–W367. doi: 10.1093/nar/gki481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seema (2020).Seema M. T cell epitope-based vaccine design for pandemic novel coronavirus 2019-nCoV. ChemRxiv. 2020 doi: 10.26434/chemrxiv.12029523.v2. [DOI] [Google Scholar]
- Shahid et al. (2020).Shahid F, Ashfaq UA, Javaid A, Khalid H. Immunoinformatics guided rational design of a next generation multi epitope based peptide (MEBP) vaccine by exploring Zika virus proteome. Infection, Genetics and Evolution. 2020;80(24):104199. doi: 10.1016/j.meegid.2020.104199. [DOI] [PubMed] [Google Scholar]
- Solanki & Tiwari (2018).Solanki V, Tiwari V. Subtractive proteomics to identify novel drug targets and reverse vaccinology for the development of chimeric vaccine against Acinetobacter baumannii. Scientific Reports. 2018;8(1):9044. doi: 10.1038/s41598-018-26689-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranzl et al. (2010).Stranzl T, Larsen MV, Lundegaard C, Nielsen M. NetCTLpan: pan-specific MHC class I pathway epitope predictions. Immunogenetics. 2010;62(6):357–368. doi: 10.1007/s00251-010-0441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer et al. (2019).Studer G, Rempfer C, Waterhouse AM, Gumienny R, Haas J, Schwede T. QMEANDisCo—distance constraints applied on model quality estimation. Bioinformatics. 2019;36(6):1765–1771. doi: 10.1093/bioinformatics/btz828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir ul Qamar et al. (2018).Tahir ul Qamar M, Bari A, Adeel MM, Maryam A, Ashfaq UA, Du X, Muneer I, Ahmad HI, Wang J. Peptide vaccine against chikungunya virus: immuno-informatics combined with molecular docking approach. Journal of Translational Medicine. 2018;16(1):298. doi: 10.1186/s12967-018-1672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir ul Qamar et al. (2019).Tahir ul Qamar M, Saleem S, Ashfaq UA, Bari A, Anwar F, Alqahtani S. Epitope-based peptide vaccine design and target site depiction against Middle East Respiratory Syndrome Coronavirus: an immune-informatics study. Journal of Translational Medicine. 2019;17(1):362. doi: 10.1186/s12967-019-2116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir ul Qamar et al. (2020).Tahir ul Qamar M, Shahid F, Ashfaq UA, Aslam S, Fatima I, Fareed MM, Zohaib A, Chen L-L. Structural modeling and conserved epitopes prediction against SARS-COV-2 structural proteins for vaccine development. Koovappally: Research Square; 2020. [Google Scholar]
- Tiwari (2020a).Tiwari V. D. novo design, retrosynthetic analysis and combinatorial synthesis of a hybrid antiviral (VTAR-01) to inhibit the interaction of SARS-CoV2 spike glycoprotein with human angiotensin-converting enzyme 2. Biology Open. 2020a;9(10):bio054056. doi: 10.1242/bio.054056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari (2020b).Tiwari V. Novel hybrid antiviral VTRRT-13V2.1 against SARS-CoV2 main protease: retro-combinatorial synthesis and molecular dynamics analysis. Heliyon. 2020b;6(10):e05122. doi: 10.1016/j.heliyon.2020.e05122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari (2021).Tiwari V. Denovo designing, retro-combinatorial synthesis, and molecular dynamics analysis identify novel antiviral VTRM1.1 against RNA-dependent RNA polymerase of SARS CoV2 virus. International Journal of Biological Macromolecules. 2021;171(4):358–365. doi: 10.1016/j.ijbiomac.2020.12.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vangone & Bonvin (2017).Vangone A, Bonvin AMJJ. PRODIGY: a contact-based predictor of binding affinity in protein-protein complexes. Bio-Protocol. 2017;7(3):e2124. doi: 10.21769/BioProtoc.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2008).Wang P, Sidney J, Dow C, Mothe B, Sette A, Peters B. A systematic assessment of MHC class II peptide binding predictions and evaluation of a consensus approach. PLOS Computational Biology. 2008;4(4):e1000048. doi: 10.1371/journal.pcbi.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederstein & Sippl (2007).Wiederstein M, Sippl MJ. ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Research. 2007;35(Web Server):W407–W410. doi: 10.1093/nar/gkm290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2020).World Health Organization . Surveillance case definitions for human infection with novel coronavirus (nCoV): interim guidance v1, January 2020. Geneva: World Health Organization; 2020. [Google Scholar]
- Wu, Leung & Leung (2020).Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(10225):689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan et al. (2020).Yan Y, Tao H, He J, Huang S-Y. The HDOCK server for integrated protein–protein docking. Nature Protocols. 2020;15(5):1829–1852. doi: 10.1038/s41596-020-0312-x. [DOI] [PubMed] [Google Scholar]
- Zhou et al. (2020).Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, Chen H-D, Chen J, Luo Y, Guo H, Jiang R-D, Liu M-Q, Chen Y, Shen X-R, Wang X, Zheng X-S, Zhao K, Chen Q-J, Deng F, Liu L-L, Yan B, Zhan F-X, Wang Y-Y, Xiao G, Shi Z-L. Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. bioRxiv. 2020 doi: 10.1101/2020.01.22.914952. [DOI] [Google Scholar]
- Zhu et al. (2020).Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W. A Novel Coronavirus from Patients with Pneumonia in China, 2019. New England Journal of Medicine. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
Raw data are available as a Supplemental File.