Figure 1.
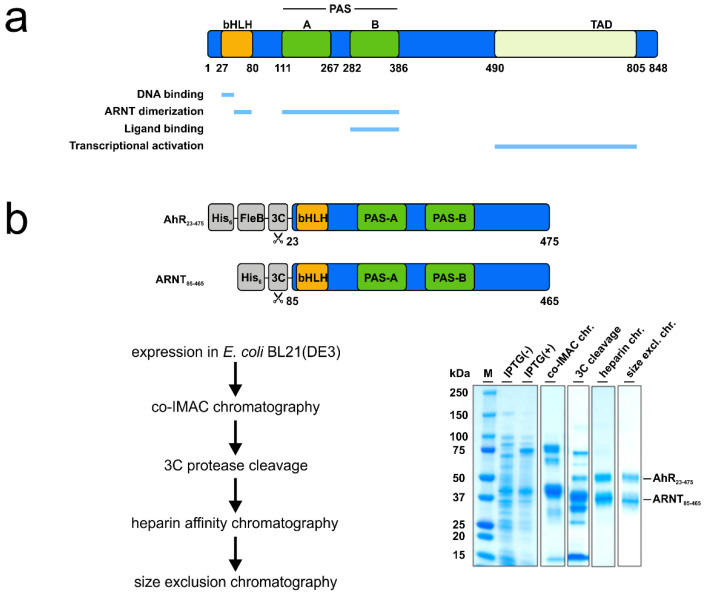
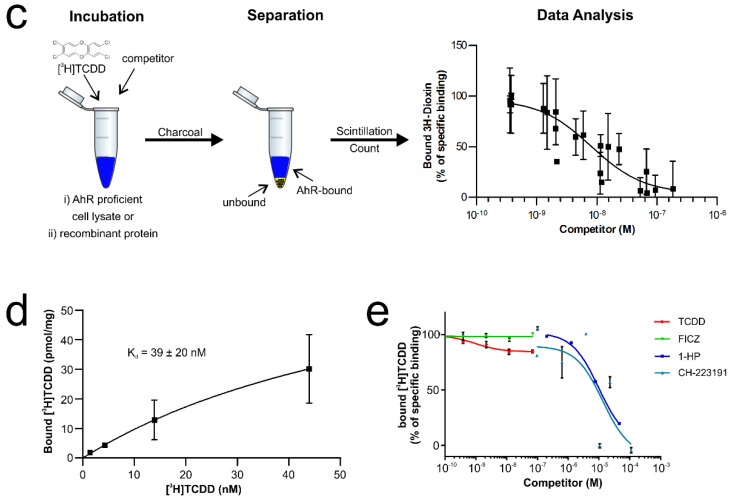
Verification of the ligand-binding properties of the recombinant hAhR-mARNT by radioligand binding assay. (a) Functional domains of the human hAhR. Numbers indicate amino acid residues displaying the relative domain boundaries. (b) Schematic representation of the AhR-ARNT protein complex purification. Both proteins were co-expressed in E. coli BL21(DE3) and purified in a 3-step purification process. Samples from different steps of the purification were analyzed by SDS-PAGE and Coomassie staining. The theoretical molecular weight of the AhR and ARNT after cleavage with 3C is 51 and 40 kDa, respectively. (c) Principle of the radioligand binding assay. AhR-deficient and -proficient liver lysates are incubated with radioactively labeled [3H]TCDD and, in case of competition assays, in presence of increasing concentrations of unlabeled competitor ligands. After incubation, charcoal is added to remove unbound tracer molecules and radioactivity of the supernatant is measured. Specific binding of radioactively labeled [3H]TCDD is calculated by fitting the experimental data with nonlinear regression after the subtraction of non-specific binding derived from AhR knockout liver lysates. The graph presented is exemplarily and is not based on experimentally derived data. (d) Saturation binding analysis of the purified AhR-ARNT receptor complex incubated with different concentrations of radioactively labeled [3H]TCDD. (e) Concentration-dependent displacement of [3H]TCDD from AhR-ARNT co-incubated with increasing concentrations of unlabeled competitor ligands. After 24 h, radioactivity in the supernatants was measured and competitive binding after NSB-subtraction was calculated. Data are mean ± SD of two independent experiments performed with triplicates.