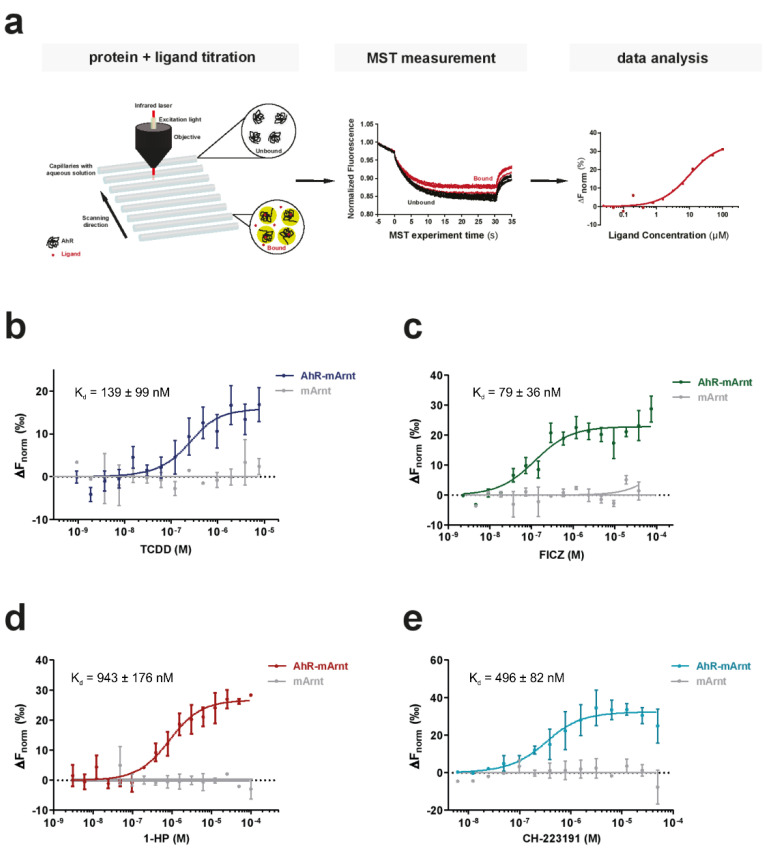
Figure 2.
Suitability of microscale thermophoresis (MST) as a sensitive method to determine AhR-ligand binding affinities. (a) The basic principle of an MST ligand binding experiment. The thermophoretic movement of the intrinsically fluorescent protein in the presence of increasing concentrations of a non-fluorescent ligand is analyzed. In the case of binding (bound, red traces), thermophoresis will differ from the unbound state and will result in a gradual change in the recorded MST traces. Plotting the changes in the normalized fluorescence as a function of the ligand concentration will yield a binding curve that can be fitted to calculate the binding affinities. (b–e) Binding of the prototypic ligand TCDD (b), the endogenous ligand FICZ (c), the bacterial pigment 1-HP (d) and the AhR inhibitor CH-223191 (e) to 250 nM recombinant AhR-ARNT complex was analyzed with label-free MST (colored lines). To verify that the measured interaction was exclusive for AhR, we examined binding to separately purified ARNT (grey line). Error bars represent the ± SD of each tested ligand concentration calculated from three independent experiments.