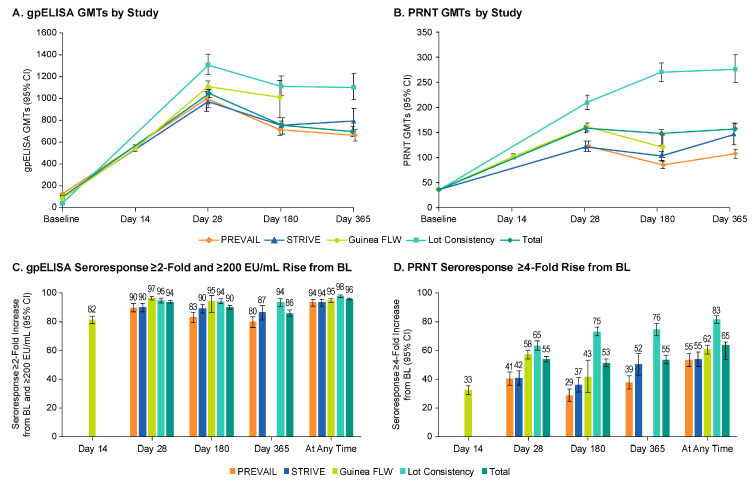
Figure 3.
Glycoprotein-enzyme-linked immunosorbent assay (GP-ELISA), plaque reduction neutralization test (PRNT), and seroresponse results by study for Partnership for Research on Ebola Virus in Liberia (PREVAIL), Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE), Guinea Front Line Worker (FLW) trial study, lot consistency study, and integrated results. (A): GP-ELISA GMTs (B): PRNT GMTs (C): GP-ELISA seroresponse ≥2-fold and ≥200 EU/mL rise from baseline (D): PRNT seroresponse ≥4-fold rise from baseline.