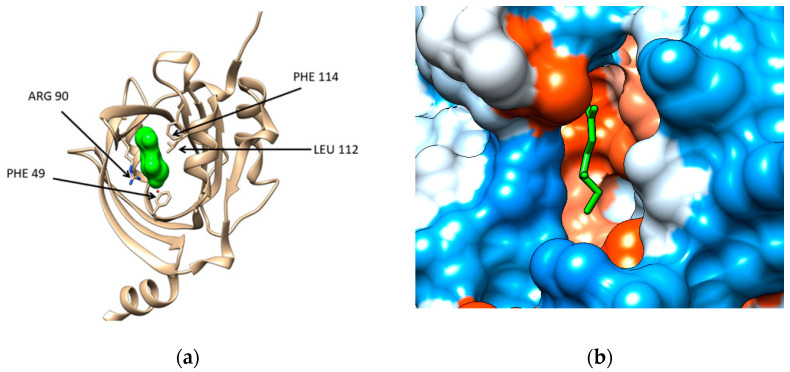
Figure 1.
(a) Structure of the human α-1-acid glycoprotein (brown ribbon) in complex with the ligand (2R)-2,3-dihydroxypropyl acetate (green solid surface), Protein data Bank (PDB) code entry 3KQ0. The residues interacting with the ligand are emphasized: PHE 49, ILE 88 (not seen being behind the ligand), ARG 90, LEU112, and PHE114; (b) Illustration of the hydrophobicity surface of the binding cavity of α-1-acid glycoprotein: blue regions are hydrophilic and orange regions are hydrophobic (dodger blue for the most hydrophilic residue to white at 0.0 and orange red for the most hydrophobic residue) and the ligand is revealed in green sticks.