Abstract
Objective
We aimed to detect the incidence and risk factors of type 2 diabetes mellitus (T2DM) development in the suburbs of Beijing.
Design
Cohort study with record linkage to incidence data.
Setting
We performed a 5-year follow-up study in a randomly selected suburban population including 1114 subjects aged ≥18 years living in the suburbs of Beijing.
Participants
118 subjects with T2DM at baseline according to the 1999 WHO criteria were excluded, and 895 subjects attended the follow-up assessment in 2012. The non-diabetic subjects at baseline were classified into two groups: normal glucose tolerance (NGT) group (n=673) and impaired glucose regulation (IGR) group(n=222). The incidence and risk factors of diabetes development in each group were investigated.
Outcome measures
A structured questionnaire about sociodemographic characteristics, height, weight, waist circumference, hip circumference, blood pressure, oral glucose tolerance test and serum lipid levels.
Results
Out of the 895 non-diabetic subjects, 67 developed diabetes with 29 in the NGT group and 38 in the IGR group, respectively, after a 5-year follow-up, producing an overall 5-year cumulative incidence of diabetes of 13%. The incidence of diabetes was 15.5 cases per 1000 person-years, 8.9 cases per 1000 person-years in the NGT group and 35.7 cases per 1000 person-years in the IGR group (p<0.01; RR 4.03; 95% CI 2.58 to 9.29). Binary logistic regression analysis showed that the risk factors for diabetes development included fasting plasma glucose (FPG) in the NGT group, and sex, the waist-to-hip ratio, FPG and diastolic blood pressure (DBP) in the IGR group.
Conclusions
During a mean follow-up of 5.0 years, the incidence of T2DM in the suburbs of Beijing was 15.5 per 1000 person-years. Early prevention of diabetes should focus on IGR subjects. Elevated FPG predicted diabetes development for both NGT and IGR subjects. Female sex, overweight/obesity and DBP are risk factors for diabetes development in IGR subjects.
Keywords: general diabetes, epidemiology, public health
Strengths and limitations of this study.
This study is a retrospective cohort survey of the incidence of type 2 diabetes mellitus and risk factors in the suburbs of Beijing.
Has a longitudinal design.
Selects a cohort by multiple-stage sampling at baseline.
Has a relatively long follow-up period.
limitations include that many variables assessed as risk factors were not updated regularly, and the data were collected only at the baseline investigation and at the end of the study.
Introduction
Diabetes mellitus (DM) is a clinical syndrome characterised by disordered glycometabolism. The lack and/or insufficiency of insulin induces metabolic disorders related to saccharides, lipids, proteins, water and electrolytes, resulting in hyperglycaemic as the main clinical feature. Long-term hyperglycaemic leads to macrovascular and microvascular complications, which may result in disability and death.1 2 Diabetes is increasingly prevalent, affecting 463 million (9.3%) adults worldwide, and these figures are expected to increase to 700 million (10.9%) by 2045.3 Type 2 DM (T2DM) accounts for over 90% of diabetes cases. Diabetes, as a leading cause of death,1 3 4 is becoming a public issue, placing a heavy burden on the healthcare system.5 In 2017 approximately 5 million adult deaths worldwide were attributed to diabetes,6 and the global healthcare expenditure associated with people with diabetes in 2019 was estimated to be US$760 billion.7
Because T2DM is usually asymptomatic, it can remain undiagnosed for many years. Almost half of all people (50.1%) living with diabetes were undiagnosed in 2019.3 Accordingly, screening for pre-diabetes/T2DM and preventing the evolution of diabetes in individuals with risk factors are essential. Currently, the risk factors related to T2DM development may include age, the body mass index (BMI), body fat distribution, a family history of diabetes, a history of cardiovascular disease (CVD), a history of gestational DM (GDM), race/ethnicity, diet, physical inactivity, hypertension, dyslipidaemia and pre-diabetes.4 8–16 Some studies have demonstrated that birth weight,17 18 income,19 socioeconomic status,20 21 working hours,21 22 occupation23 and genetic factors24–26 might also contribute to T2DM development.
In addition to the process of urbanisation, the population ageing, changes in lifestyle and increasing prevalence of obesity and overweight, the prevalence of diabetes in China has increased over the past three decades. The prevalence of diabetes in China was 0.67% in 1980,27 2.12% in 1995,28 5.5% in 2001,29 9.7% in 2008,30 11.6% in 201031 and 10.9% in 2013.32 As the capital city, limited information is available regarding the incidence of diabetes and pre-diabetes in Beijing. Certain studies have suggested that different diabetic risk patterns might exist in Asian populations.33–36
This study aimed to determine the incidence of and risk factors for T2DM among Chinese adults in the suburbs of Beijing.
Materials and methods
Study population
The study population comprised residents living in the suburbs (Huairou, Pinggu and Hepingli) of Beijing. We used a multistage, stratified sampling method to randomly select the three suburbs from the Beijing countryside.30 The details of its sampling methods have been described previously.30 Individuals aged 18 years or older who were willing to participate and provided informed consent were eligible to participate in the study. People with a history of diabetes were excluded from the study. Pregnant women were also excluded from the study.
We performed a 5-year retrospective cohort study. The baseline survey occurred from June 2007 to September 2008 using a random sampling method with a follow-up examination from May to July 2012. All the subjects were asked to undergo a personal interview, physical examination and blood test (including an oral glucose tolerance test (OGTT)). Subjects diagnosed as diabetes by OGTT at baseline were excluded. The non-diabetic subjects at baseline were divided into the normal glucose tolerance (NGT) group and impaired glucose regulation (IGR) group according to OGTT. We analysed the incidence and risk factors of diabetes development in each group. DM was defined according to the 1990 WHO criteria or self-reported prior diagnosis of diabetes with current medication use. IGR was determined if the subjects had a fasting plasma glucose (FPG) level of 6.1–6.9 mmol/L and/or a 2-hour plasma glucose level of 7.8–11.0 mmol/L. NGT was defined as FPG less than 6.0 mmol/L and a 2-hour plasma glucose level less than 11.1 mmol/L.
Data collection
Sociodemographic characteristics
The data were collected by trained staff using a structured questionnaire via a face-to-face interview to assess general information (sex, age, nationality, education status, occupation and per capita income), personal history (smoking history, alcohol history, physical activity and dietary habits), family history (DM, hypertension, hyperlipidaemia, myocardial infarction, stroke and obesity), and history of current illness (diabetes, hypertension, hyperlipidaemia, cardiovascular, CVDs and kidney diseases). Subjects who were diagnosed with T2DM between recruitment and the end of follow-up were asked to report the date of diagnosis.
Anthropometric measurements
The subjects were examined for height, weight, waist circumference (WC), hip circumference (HC) and blood pressure. All the subjects were asked to remove their shoes, socks, hats and coats, stand erectly and look forward with their arms relaxed and their heels together. Height was measured in centimetres using a height bar, and weight was measured in kilograms using a digital weighing scale. The WC is the circumference of the waist at the horizontal line of the umbilicus measured in centimetres using a measuring tape, and the HC is the circumference of hips at the horizontal line of the anterior superior spine measured in centimetres using a measuring tape. The BMI was calculated as weight in kilograms divided by the square of height in metres (kg/m2), while the waist-to-hip ratio (WHR) was calculated as WC (cm) divided by HC (cm). The blood pressure values used were an average of three measurements, which were measured 2 min apart using a mercury sphygmomanometer. The subjects were asked to stop smoking and consuming alcohol the day before the examination and to sit quietly in a chair for at least 5 min before the measurement with their arms bare and placed at the chest level. All the clinical staff members were trained to measure blood pressure and obtain anthropometric measurements.
Laboratory examination
All the subjects were invited to undergo a blood test (including an OGTT) at baseline. The non-diabetic subjects at baseline undergo a secondary blood test (including an OGTT) at the end of follow-up. Venous blood samples after 8–14 hours of fasting were obtained from the subjects to measure FPG, total cholesterol (TC), triglycerides (TGs), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C). Venous blood samples, after a 75 g oral glucose load, were obtained to measure the 2-hour plasma glucose (PG2h). The subjects were instructed to maintain normal physical activity without dietary limit (the intake of saccharides should be no less than 150 g per day) before proceeding with the OGTT.
Statistical analysis
We used the P-P plot to test the normality of the numerical variables. The differences in continuous and categorical variables between the NGT and IGR groups were examined using t test and the χ2 test statistic, respectively. Kaplan-Meier survival estimates were used to calculate the 5-year cumulative incidence of T2DM. The log-rank test was used to compare the survival curves. The Mantel-Haenszel χ2 test of trends was used to analyse the ordinal data. Binary logistic regression analyses were used to estimate the OR and 95% CI for diabetes development. All the analyses were performed using SPSS statistical software V.17.0, and a p<0.05 was considered statistically significant.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Results
Characteristics of the study population at baseline
In total, 1114 residents participated in the study, and 1014 subjects completed the follow-up, with an overall response rate of 91.0%. After eliminating 118 subjects who were diagnosed with diabetes by OGTT at baseline and one subject with severe data deficiency, 895 subjects (308 men and 587 women) with a mean age of 48.1±11.9 years were included in the analysis, with 673 in the NGT group and 222 in the IGR group. The baseline characteristics of the 895 subjects in the study are shown in table 1. Continuous variables were expressed as means±SD and were examined using a t-test, while categorical variables were expressed as percentages and were examined using the χ2 test. No significant difference was found between the NGT group and the IGR group in sex, height, family history of T2DM, smoking history, alcohol history, exercise time, diastolic blood pressure (DBP) or HDL-C. Age, weight, BMI, WC, WHR, systolic blood pressure, FPG, PG2h, TGs, TC and LDL-C in the IGR group were significantly higher than those in the NGT group.
Table 1.
Comparison of the characteristics of the NGT and IGR subjects at baseline
| Variable at baseline | NGT group (n=673) | IGR group (n=222) | T or χ², P value |
| Sex (% male) | 34.9 | 32.9 | 0.306, 0.625 |
| Age (years) | 46.75±11.57 | 52.01±12.03 | −5.813, <0.001 |
| Height (cm) | 161.85±7.94 | 161.18±7.83 | 1.092, 0.275 |
| Weight (kg) | 64.32±10.75 | 67.49±11.05 | −3.777, <0.001 |
| BMI (kg/m2) | 24.52±3.40 | 25.93±3.50 | −5.332, <0.001 |
| WC (cm) | 83.04±9.75 | 86.82±9.20 | −5.075, <0.001 |
| WHR | 0.87±0.07 | 0.89±0.06 | −3.495, <0.001 |
| SBP (mm Hg) | 119.71±18.37 | 125.18±20.13 | −3.582, <0.001 |
| DBP (mm Hg) | 78.02±11.21 | 79.75±12.66 | −1.843, 0.066 |
| FPG (mmol/L) | 5.25±0.42 | 5.82±0.61 | −15.875, <0.001 |
| PG2h (mmol/L) | 5.90±1.01 | 8.29±1.52 | −26.692, <0.001 |
| TGs (mmol/L) | 1.42±1.17 | 1.61±1.08 | −2.165, 0.031 |
| TC (mmol/L) | 4.56±0.92 | 4.84±0.95 | −3.865, <0.001 |
| HDL-C (mmol/L) | 1.35±0.34 | 1.34±0.36 | 0.464, 0.643 |
| LDL-C (mmol/L) | 2.77±0.77 | 3.08±0.81 | −4.648, <0.001 |
| Exercise duration (hours) | 5.85±11.81 | 5.59±8.06 | 0.305, 0.761 |
| FH of T2DM (%) | 15.5 | 21.4 | 3.722, 0.068 |
| Smoking history (%) | 22.4 | 20.7 | 0.286, 0.641 |
| Alcohol history (%) | 27.9 | 28.8 | 0.076, 0.796 |
| Years of follow-up (year) | 4.85±0.32 | 4.79±0.56 | 2.081, 0.038 |
Exercise duration is expressed as hours per week.
BMI, body mass index; DBP, diastolic blood pressure; FH, family history; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; IGR, impaired glucose regulation; LDL-C, low-density lipoprotein cholesterol; NGT, normal glucose tolerance; PG2h, 2 hour plasma glucose; SBP, systolic blood pressure; TC, total cholesterol; T2DM, type 2 diabetes mellitus; TGs, triglycerides; WC, waist circumference; WHR, waist-to-hip ratio.
Incidence of T2DM
During the follow-up, T2DM developed in 67 subjects, 29 in the NGT group and 38 in the IGR group, resulting in T2DM onset rates of 15.45, 8.86 and 35.67 per 1000 person-years in the total study population, NGT group and IGR group, respectively (table 2). The difference in the incidence of T2DM between the NGT group and IGR group was statistically significant (χ²=37.38; p<0.01; RR 4.03; 95% CI 2.58 to 9.29).
Table 2.
Incidence of T2DM during the follow-up
| Cases of diabetes | Follow-up (person-years) | Incidence (per 1000 person-years) | |
| NGT group | 29 | 3271.5 | 8.86 |
| IGR group | 38 | 1065.3 | 35.67 |
| Total | 67 | 4336.8 | 15.45 |
IGR, impaired glucose regulation; NGT, normal glucose tolerance; T2DM, type 2 diabetes mellitus.
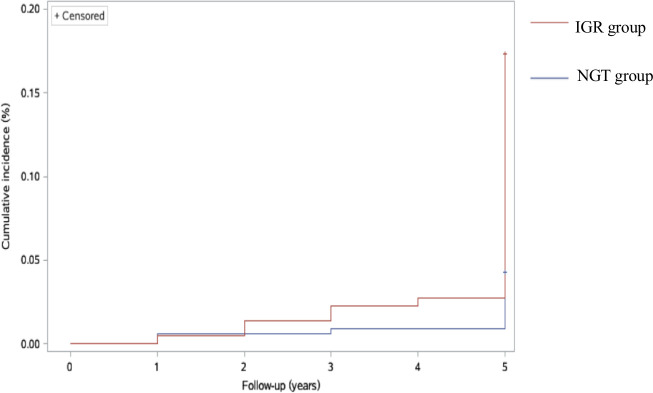
The 5-year cumulative incidence of T2DM was 13%, with 10% in the NGT group and 20% in the IGR group. The mean follow-up duration was 5.008 years (SE 0.013; 95% CI 4.982 to 5.033), with 5.003 years (SE 0.012; 95% CI 5.010 to 5.056) in the NGT group and 4.933 years (SE 0.038; 95% CI 4.859 to 5.007) in the IGR group. Using the log-rank test, we found that the cumulative incidence of T2DM in the IGR group was significantly higher than that in the NGT group (χ²=36.905; p<0.0001). The results of the Kaplan-Meier survival analyses are shown in figure 1.
Figure 1.
Cumulative incidence of T2DM in the NGT and IGR groups (log-rank test: χ²=36.905; p<0.0001). IGR, impaired glucose regulation; NGT, normal glucose tolerance; T2DM, type 2 diabetes mellitus.
Analyses of the risk factors for diabetes development
The results of binary logistic regression analyses for T2DM development in the NGT and IGR groups are shown in table 3. FPG contributed to T2DM development in the NGT group, and the OR was 6.111 (95% CI 1.379 to 27.070; p=0.017). Sex, WHR, DBP and FPB contributed to T2DM development in the IGR group, and the ORs were 7.293 (95% CI 1.074 to 49.549; p=0.042), 2.874E8 (95% CI 8.386 to 9.847E15; p=0.028), 1.068 (95% CI 1.009 to 1.130; p=0.024) and 7.243 (95% CI 2.314 to 22.673; p=0.001), respectively. The increase in exercise time slightly decreased the risk of T2DM in the IGR group, with an OR of 0.923 (95% CI 0.847 to 1.005; p=0.066).
Table 3.
Multivariate logistic regression model for T2DM development
| NGT group | IGR group | |||
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Sex | 0.375 (0.067 to 2.103) | 0.265 | 7.293 (1.074 to 49.549) | 0.042 |
| Age (years) | 1.013 (0.962 to 1.068) | 0.615 | 1.019 (0.964 to 1.077) | 0.499 |
| Weight (kg) | 1.044 (0.927 to 1.175) | 0.478 | 1.056 (0.929 to 1.200) | 0.402 |
| BMI (kg/m2) | 0.934 (0.653 to 1.336) | 0.710 | 0.971 (0.661 to 1.427) | 0.881 |
| WC (cm) | 1.073 (0.936 to 1.230) | 0.311 | 0.904 (0.748 to 1.092) | 0.295 |
| WHR | 0.006 (0.000 to 7058.081) | 0.473 | 2.874E8 (8.386 to 9.847E15) | 0.028 |
| SBP (mm Hg) | 1.013 (0.976 to 1.052) | 0.492 | 0.992 (0.958 to 1.026) | 0.637 |
| DBP (mm Hg) | 0.955 (0.890 to 1.024) | 0.198 | 1.068 (1.009 to 1.130) | 0.024 |
| FPG (mmol/L) | 6.111 (1.379 to 27.070) | 0.017 | 7.243 (2.314 to 22.673) | 0.001 |
| TC (mmol/L) | 2.719 (0.917 to 8.067) | 0.071 | 0.814 (0.141 to 4.699) | 0.818 |
| TGs (mmol/L) | 0.996 (0.589 to 1.684) | 0.989 | 0.926 (0.491 to 1.749) | 0.813 |
| HDL-C (mmol/L) | 2.536 (0.519 to 12.395) | 0.250 | 0.622 (0.071 to 5.460) | 0.669 |
| LDL-C (mmol/L) | 0.604 (0.206 to 1.769) | 0.358 | 1.070 (0.181 to 6.321) | 0.941 |
| Exercise duration (hours) | 1.013 (0.947 to 1.084) | 0.707 | 0.923 (0.847 to 1.005) | 0.066 |
| FH of T2DM | 1.868 (0.492 to 7.090) | 0.358 | 2.062 (0.656 to 6.478) | 0.215 |
| Smoking history | 0.766 (0.186 to 3.162) | 0.713 | 1.591 (0.346 to 7.317) | 0.551 |
| Alcohol history | 0.726 (0.202 to 2.610) | 0.624 | 1.685 (0.458 to 6.203) | 0.432 |
Sex is defined as male (n=1) or female (n=2), FH of T2DM is defined as no (n=1) or yes (n=2), smoking history was defined as no (n=1) or yes (n=2), and alcohol history was defined as no (n=1) or yes (n=2). Exercise duration is expressed as hours per week.
BMI, body mass index; DBP, diastolic blood pressure; FH, family history; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; IGR, impaired glucose regulation; LDL-C, low-density lipoprotein cholesterol; NGT, normal glucose tolerance; SBP, systolic blood pressure; TC, total cholesterol; T2DM, type 2 diabetes mellitus; TGs, triglycerides; WC, waist circumference; WHR, waist-to-hip ratio.
Discussion
The prevalence of DM has been increasing markedly in recent decades. Among adults aged 20–79 years in 2019, there were an estimated 463 million cases of diabetes.3 However, the striking prevalence may be partly attributable to the increase in population and prolongation of the life span.37 Accordingly, we studied the growing trend of T2DM by investigating the cumulative incidence of T2DM. Our results showed that 15.45 new cases of diabetes per 1000 people-years were diagnosed during the 5 years observation period (2007–2012). This marked trend is consistent with diabetes incidence rates worldwide, such as those described in the ATTICA study in Greece (12.9 cases per 1000 person-years from 2002 to 2012)38 and those described in a study conducted in Mexico (12.7 cases per 1000 person-years from 1990 to 2008).39 The incidence rate found in our study is lower than that in a study of Pima Indians (23.5 cases per 1000 person-years during 1991–2003)40 and in a study conducted in northern Spain (95.2 cases per 1000 person-years during 1998–2005)41 and is higher than that observed in a study conducted in Iran (10.6 cases per 1000 person-years from 1999 to 2011)42 and in the Surveillance, Prevention, and Management of Diabetes Mellitus(SUPREME-DM) project in the USA (11.5 cases per 1000 person-years from 2006 to 2011).43 The T2DM incidence in the suburbs of Beijing is higher than that identified in the investigation conducted by Wang et al44 in a Chinese population in 2010 (9.5 cases per 1000 person-years in men and 9.2 in women). This difference may partly be due to the relatively higher standard of living in the suburbs of Beijing.
Diabetes development is based on the interaction between genes and lifestyle and environmental factors.45 46 Compared with the NGT group that had an incidence of 8.86 cases per 1000 person-years, the IGR group (35.67 cases per 1000 person-years) had a higher incidence rate (RR 4.03; 95% CI 2.58 to 9.29; p<0.01). In our study, diabetes development was mainly the consequence of elevated FPG in the NGT group. Diabetes development was mainly associated with sex (female), abnormal WHR, elevated DBP and elevated FPG in the IGR group. The increase in the FPG level was a strong predictor of diabetes development in both the NGT group and IGR group, with 6.111-fold and 7.243-fold increased risk per unit increase, respectively.
In our study, the WHR measurement was found to predict diabetes risk. Some studies have declared that WHR, as an obesity indicator, is superior to BMI and WC.47 48 A meta-analysis demonstrated that either BMI or WC (WHR) predicted or was independently associated with type 2 diabetes, regardless of the controversial findings regarding which was better.49
Age and family history of diabetes are considered risk factors for diabetes development.4 8–16 However, we found only an increasing trend of diabetes development in the elderly population and subjects with a family history of diabetes (p>0.05). This finding might be related to the short period of follow-up and the subjects’ unawareness of their family history of diabetes.
There is evidence that T2DM can be prevented in high-risk individuals by a lifestyle programme of regular exercise.50 51 Surprisingly, our study found that exercise did not exert a beneficial effect on diabetes incidence, a finding similar to that in the ATTICA study.38 52 However, we found a decreasing trend in the diabetes incidence with increasing exercise time. The increase in exercise time slightly decreased the risk of T2DM in the IGR group, with an OR of 0.923 (95% CI 0.847 to 1.005; p=0.066).
The strength of our study is that it was based on a cohort selected by multiple-stage sampling at baseline with a relatively long follow-up period. A limitation is that many variables assessed as risk factors were not updated regularly, and might have changed during the follow-up period. Second, the follow-up could not be random due to the limitations of the conditions. Additionally, the data were collected only at the baseline investigation and at the end of the study.
Conclusions
During a mean follow-up of 5.0 years, the incidence of T2DM in the suburbs of Beijing was 15.45 cases per 1000 person-years, which was relatively higher than that in many other areas worldwide. Compared with the NGT subjects, the IGR subjects were more susceptible to T2DM. To prevent diabetes development, all the subjects should be evaluated for elevated FPG. Additionally, sex, WHR and DBP were predictors of T2DM in IGR subjects.
The findings in this study provide new data on the incidence of T2DM in the Beijing area. The predictors of T2DM reported in the present study may be conducive to formulating a protocol for diabetes prevention.
Supplementary Material
Footnotes
LX and XZ contributed equally.
Contributors: Conceptualisation, BZ; Data curation, HZ; Formal analysis, HZ; Investigation, LX and XZ; Methodology, BZ; Project administration, LX, XZ and BZ; Writing—original draft, LX and XZ; Writing—review and editing, BZ. All authors read and approved the final manuscript, as well as the submission of this work. BZ supervised and managed the data. BZ is the guarantor of this work.
Funding: The study was supported by grants from the 11th Five-year National Science and Technology Support Program (2009BAI80B04).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Ethics approval: Ethical approval was obtained from the Ethics Committee of Clinical Trials of Drugs/Devices in China-Japan Friendship Hospital (2011-049).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as online supplemental information.
References
- 1.GBD 2013 Mortality and Causes of Death Collaborators . Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the global burden of disease study 2013. Lancet 2015;385:117–71. 10.1016/S0140-6736(14)61682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong E, Backholer K, Gearon E, et al. Diabetes and risk of physical disability in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2013;1:106–14. 10.1016/S2213-8587(13)70046-9 [DOI] [PubMed] [Google Scholar]
- 3.Sinclair A, Saeedi P, Kaundal A, et al. Diabetes and global ageing among 65-99-year-old adults: Findings from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 2020;162:108078. 10.1016/j.diabres.2020.108078 [DOI] [PubMed] [Google Scholar]
- 4.Mc Donald Posso AJ, Bradshaw Meza RA, Mendoza Morales EA, et al. Diabetes in Panama: epidemiology, risk factors, and clinical management. Ann Glob Health 2015;81:754–64. 10.1016/j.aogh.2015.12.014 [DOI] [PubMed] [Google Scholar]
- 5.Bommer C, Sagalova V, Heesemann E, et al. Global economic burden of diabetes in adults: projections from 2015 to 2030. Diabetes Care 2018;41:963–70. 10.2337/dc17-1962 [DOI] [PubMed] [Google Scholar]
- 6.Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 2018;138:271–81. 10.1016/j.diabres.2018.02.023 [DOI] [PubMed] [Google Scholar]
- 7.Williams R, Karuranga S, Malanda B, et al. Global and regional estimates and projections of diabetes-related health expenditure: results from the International diabetes Federation diabetes atlas, 9th edition. Diabetes Res Clin Pract 2020;162:108072. 10.1016/j.diabres.2020.108072 [DOI] [PubMed] [Google Scholar]
- 8.Weber MB, Oza-Frank R, Staimez LR, et al. Type 2 diabetes in Asians: prevalence, risk factors, and effectiveness of behavioral intervention at individual and population levels. Annu Rev Nutr 2012;32:417–39. 10.1146/annurev-nutr-071811-150630 [DOI] [PubMed] [Google Scholar]
- 9.Pérez CM, Soto-Salgado M, Suárez E, et al. High prevalence of diabetes and prediabetes and their coexistence with cardiovascular risk factors in a Hispanic community. J Immigr Minor Health 2015;17:1002–9. 10.1007/s10903-014-0025-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aljoudi AS, Taha AZA. Knowledge of diabetes risk factors and preventive measures among Attendees of a primary care center in eastern Saudi Arabia. Ann Saudi Med 2009;29:15–19. 10.4103/0256-4947.51813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Diabetes Association . 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care 2018;41:S13–27. 10.2337/dc18-S002 [DOI] [PubMed] [Google Scholar]
- 12.Fletcher B, Gulanick M, Lamendola C. Risk factors for type 2 diabetes mellitus. J Cardiovasc Nurs 2002;16:17–23. 10.1097/00005082-200201000-00003 [DOI] [PubMed] [Google Scholar]
- 13.Karter AJ, Schillinger D, Adams AS, et al. Elevated rates of diabetes in Pacific Islanders and Asian subgroups: the diabetes study of northern California (distance). Diabetes Care 2013;36:574–9. 10.2337/dc12-0722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harjo TC, Perez A, Lopez V, et al. Prevalence of diabetes and cardiovascular risk factors among California native American adults compared to other ethnicities: the 2005 California health interview survey. Metab Syndr Relat Disord 2011;9:49–54. 10.1089/met.2010.0043 [DOI] [PubMed] [Google Scholar]
- 15.Akter S, Rahman MM, Abe SK, et al. Prevalence of diabetes and prediabetes and their risk factors among Bangladeshi adults: a nationwide survey. Bull World Health Organ 2014;92:204–13. 10.2471/BLT.13.128371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amarasinghe S, Balakumar S, Arasaratnam V. Prevalence and risk factors of diabetes mellitus among adults in Jaffna district. Ceylon Med J 2015;60:107–10. 10.4038/cmj.v60i3.8191 [DOI] [PubMed] [Google Scholar]
- 17.Pettitt DJ, Jovanovic L. Low birth weight as a risk factor for gestational diabetes, diabetes, and impaired glucose tolerance during pregnancy. Diabetes Care 2007;30 Suppl 2:S147–9. 10.2337/dc07-s207 [DOI] [PubMed] [Google Scholar]
- 18.Harder T, Rodekamp E, Schellong K, et al. Birth weight and subsequent risk of type 2 diabetes: a meta-analysis. Am J Epidemiol 2007;165:849–57. 10.1093/aje/kwk071 [DOI] [PubMed] [Google Scholar]
- 19.Barker L, Crespo R, Gerzoff RB, et al. Residence in a distressed County in Appalachia as a risk factor for diabetes, behavioral risk factor surveillance system, 2006-2007. Prev Chronic Dis 2010;7:A104. [PMC free article] [PubMed] [Google Scholar]
- 20.Espelt A, Arriola L, Borrell C, et al. Socioeconomic position and type 2 diabetes mellitus in Europe 1999-2009: a Panorama of inequalities. Curr Diabetes Rev 2011;7:148–58. 10.2174/157339911795843131 [DOI] [PubMed] [Google Scholar]
- 21.Kivimäki M, Virtanen M, Kawachi I, et al. Long working hours, socioeconomic status, and the risk of incident type 2 diabetes: a meta-analysis of published and unpublished data from 222 120 individuals. Lancet Diabetes Endocrinol 2015;3:27–34. 10.1016/S2213-8587(14)70178-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bannai A, Yoshioka E, Saijo Y, et al. The risk of developing diabetes in association with long working hours differs by shift work schedules. J Epidemiol 2016;26:481–7. 10.2188/jea.JE20150155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honda T, Kuwahara K, Nakagawa T, et al. Leisure-Time, occupational, and commuting physical activity and risk of type 2 diabetes in Japanese workers: a cohort study. BMC Public Health 2015;15:1004. 10.1186/s12889-015-2362-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma RCW, Hu C, Tam CH, et al. Genome-wide association study in a Chinese population identifies a susceptibility locus for type 2 diabetes at 7q32 near Pax4. Diabetologia 2013;56:1291–305. 10.1007/s00125-013-2874-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grarup N, Sandholt CH, Hansen T, et al. Genetic susceptibility to type 2 diabetes and obesity: from genome-wide association studies to rare variants and beyond. Diabetologia 2014;57:1528–41. 10.1007/s00125-014-3270-4 [DOI] [PubMed] [Google Scholar]
- 26.Florez JC, Jablonski KA, Bayley N, et al. Tcf7L2 polymorphisms and progression to diabetes in the diabetes prevention program. N Engl J Med 2006;355:241–50. 10.1056/NEJMoa062418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NDrC G. [A mass survey of diabetes mellitus in a population of 300,000 in 14 provinces and municipalities in China (author's transl)]. Zhonghua Nei Ke Za Zhi 1981;20:678–83. [PubMed] [Google Scholar]
- 28.Pan XR, Yang WY, Li GW, et al. Prevalence of diabetes and its risk factors in China, 1994. National diabetes prevention and control cooperative group. Diabetes Care 1997;20:1664–9. 10.2337/diacare.20.11.1664 [DOI] [PubMed] [Google Scholar]
- 29.Chan JCN, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 2009;301:2129–40. 10.1001/jama.2009.726 [DOI] [PubMed] [Google Scholar]
- 30.Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med 2010;362:1090–101. 10.1056/NEJMoa0908292 [DOI] [PubMed] [Google Scholar]
- 31.Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013;310:948–59. 10.1001/jama.2013.168118 [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA 2017;317:2515–23. 10.1001/jama.2017.7596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narayan KMV, Aviles-Santa L, Oza-Frank R, et al. Report of a national heart, lung, and blood Institute workshop: heterogeneity in cardiometabolic risk in Asian Americans in the U.S. opportunities for research. J Am Coll Cardiol 2010;55:966–73. 10.1016/j.jacc.2009.07.075 [DOI] [PubMed] [Google Scholar]
- 34.Oza-Frank R, Ali MK, Vaccarino V, et al. Asian Americans: diabetes prevalence across U.S. and world Health organization weight classifications. Diabetes Care 2009;32:1644–6. 10.2337/dc09-0573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma RCW, Chan JCN. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci 2013;1281:64–91. 10.1111/nyas.12098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gujral UP, Pradeepa R, Weber MB, et al. Type 2 diabetes in South Asians: similarities and differences with white Caucasian and other populations. Ann N Y Acad Sci 2013;1281:51–63. 10.1111/j.1749-6632.2012.06838.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gulland A. Global life expectancy has risen, reports who. BMJ 2014;348:g3369. 10.1136/bmj.g3369 [DOI] [PubMed] [Google Scholar]
- 38.Koloverou E, Panagiotakos DB, Pitsavos C, et al. 10-Year incidence of diabetes and associated risk factors in Greece: the Attica study (2002-2012). Rev Diabet Stud 2014;11:181–9. 10.1900/RDS.2014.11.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.González-Villalpando C, Dávila-Cervantes CA, Zamora-Macorra M, et al. Incidence of type 2 diabetes in Mexico: results of the Mexico City diabetes study after 18 years of follow-up. Salud Publica Mex 2014;56:11–17. 10.21149/spm.v56i1.7318 [DOI] [PubMed] [Google Scholar]
- 40.Pavkov ME, Hanson RL, Knowler WC, et al. Changing patterns of type 2 diabetes incidence among Pima Indians. Diabetes Care 2007;30:1758–63. 10.2337/dc06-2010 [DOI] [PubMed] [Google Scholar]
- 41.Valdés S, Botas P, Delgado E, et al. Population-Based incidence of type 2 diabetes in northern Spain: the asturias study. Diabetes Care 2007;30:2258–63. 10.2337/dc06-2461 [DOI] [PubMed] [Google Scholar]
- 42.Derakhshan A, Sardarinia M, Khalili D, et al. Sex specific incidence rates of type 2 diabetes and its risk factors over 9 years of follow-up: Tehran lipid and glucose study. PLoS One 2014;9:e102563. 10.1371/journal.pone.0102563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nichols GA, Schroeder EB, Karter AJ, et al. Trends in diabetes incidence among 7 million insured adults, 2006-2011: the SUPREME-DM project. Am J Epidemiol 2015;181:32–9. 10.1093/aje/kwu255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang C, Li J, Xue H, et al. Type 2 diabetes mellitus incidence in Chinese: contributions of overweight and obesity. Diabetes Res Clin Pract 2015;107:424–32. 10.1016/j.diabres.2014.09.059 [DOI] [PubMed] [Google Scholar]
- 45.Murea M, Ma L, Freedman BI. Genetic and environmental factors associated with type 2 diabetes and diabetic vascular complications. Rev Diabet Stud 2012;9:6–22. 10.1900/RDS.2012.9.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46., Langenberg C, Sharp S, et al. , InterAct Consortium . Design and cohort description of the interact project: an examination of the interaction of genetic and lifestyle factors on the incidence of type 2 diabetes in the EPIC study. Diabetologia 2011;54:2272–82. 10.1007/s00125-011-2182-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao X, Zhu X, Zhang H, et al. Prevalence of diabetes and predictions of its risks using anthropometric measures in Southwest rural areas of China. BMC Public Health 2012;12:821. 10.1186/1471-2458-12-821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta R, Rastogi P, Sarna M, et al. Body-Mass index, waist-size, waist-hip ratio and cardiovascular risk factors in urban subejcts. J Assoc Physicians India 2007;55:621–7. [PubMed] [Google Scholar]
- 49.Qiao Q, Nyamdorj R. Is the association of type II diabetes with waist circumference or waist-to-hip ratio stronger than that with body mass index? Eur J Clin Nutr 2010;64:30–4. 10.1038/ejcn.2009.93 [DOI] [PubMed] [Google Scholar]
- 50.Dela F, Prats C, Helge JW. Exercise interventions to prevent and manage type 2 diabetes: physiological mechanisms. Med Sport Sci 2014;60:36–47. 10.1159/000357334 [DOI] [PubMed] [Google Scholar]
- 51.Teixeira-Lemos E, Nunes S, Teixeira F, et al. Regular physical exercise training assists in preventing type 2 diabetes development: focus on its antioxidant and anti-inflammatory properties. Cardiovasc Diabetol 2011;10:12. 10.1186/1475-2840-10-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Panagiotakos DB, Pitsavos C, Skoumas Y, et al. Five-Year incidence of type 2 diabetes mellitus among cardiovascular disease-free Greek adults: findings from the Attica study. Vasc Health Risk Manag 2008;4:691–8. 10.2147/VHRM.S2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.