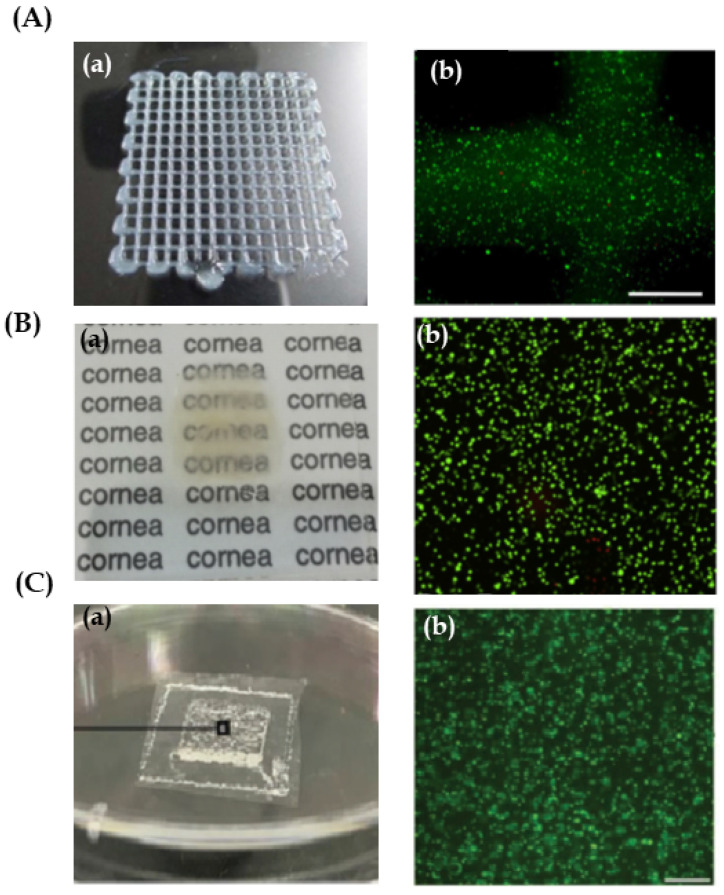
Figure 5.
(A) Reproduced with permission from [45], Nature, 2016. Cornea epithelial structures: (a) Top view of a 3D human corneal epithelial cells/gelatin/alginate construct. (b) Epithelial cell viability after extrusion bioprinting by live/dead staining, showing live (green) and dead (red) cells. Scale bar 500 μm. (B) Reproduced with permission from [32], Elsevier, 2018. Cornea stroma and epithelium structure. (a) 3D bio-printed cornea from human embryonic stem cells and human adipose tissue derived stem cells fabricated onto supportive membrane using laser-assisted bioprinting. This shows moderate transparency. (b) Cell viability of human embryonic stem cells seven days after printing shown with live-dead-staining. Live cells are visualized with green and dead cells with red. Scale bar 1 mm. (C) Reproduced with permission from [46], Wiley, 2019. Cornea endothelium structures. (a) Image of the bio-printed genetically modified human corneal endothelial cells (HCECs)/gelatin scaffold on an amniotic membrane. (b) The seeded live HCECs were densely and evenly distributed just after bioprinting. Scale bar: 500 μm.