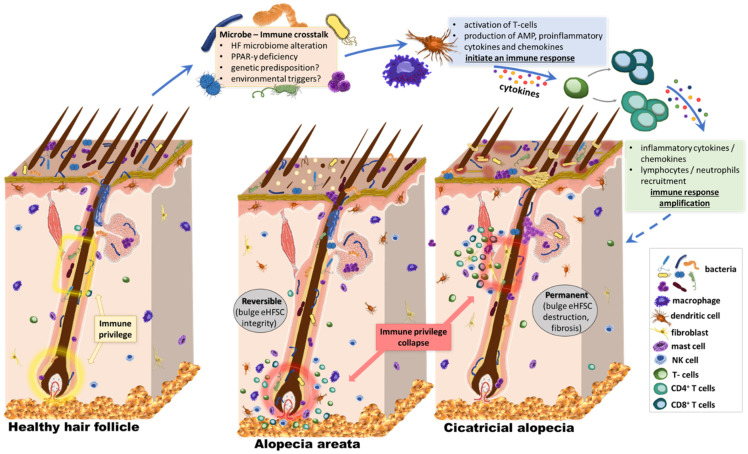
Figure 2.
Our hypothesis. The effect of the microbiome on HF stem cell niche protection and survival. The skin surface and follicular openings are recognized sites of rich microbial colonization and intense immune activation, with cross-talk across the skin barrier. The bacterial microbiome extends below the infundibulum, with the confirmed presence of various species and high abundance of Corynebacterium and Staphylococcus. It is in close proximity to structures of the immune privileged status, which are essential for the hair cycle. Several preliminary studies suggest that external stimuli could affect the state of activation of the HF immune system. Lower sections of the HF are protected from immune cell infiltration under healthy conditions, the so-called “immune-privileged” areas. This includes the bulge, where a stem cell niche is found, and the bulb, where cells divide and grow to build the new hair. However, both regions are sites of intense inflammatory infiltrate in inflammatory hair diseases like primary cicatricial alopecia and alopecia areata. Alterations in the HF microbiome or the penetration depth of microbial material could be related to homeostasis, modulation of cutaneous immune reactions, and inflammatory processes along the HF. The localization of this involvement with the immune system is critical, affecting the possibility of regrowth after subsidence of inflammation. When the stem cell niche is attacked, like in cicatricial alopecia, patients suffer permanent, scarring hair loss. On the other hand, AA pathogenesis involves inflammation of the peribulbar region, allowing a possible hair regrowth. The role of microbiota in the pathogenesis of alopecia is proposed, but in ways more complex than just bacterial abundance of specific species in the area. HF, hair follicle.