Figure 2.
Placental and breastmilk transfer of vaccine-induced SARS-CoV-2 antibodies
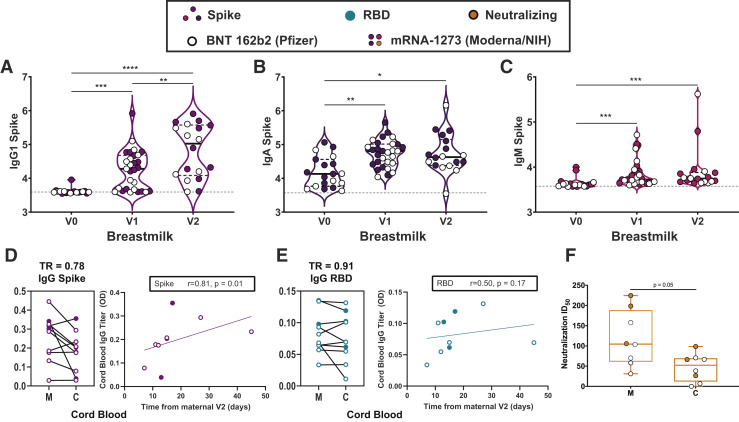
A–C, Violin plots show the log10 transformed mean fluorescence intensity (MFI) for (A) IgG1-, (B) IgA-, and (C) IgM spike–specific breastmilk titers across V0, V1, and V2 time points. Differences across time points were assessed with repeated measures mixed-effects model followed by post hoc Tukey’s multiple comparisons test. Participants who received BNT 162b2 from Pfizer/BioNTech are depicted as open circles, and participants who received mRNA-1273 from Moderna/NIH are depicted as closed circles. The asterisk indicates P<.05, the double asterisk indicates P<.01, the triple asterisk indicates P<.001, and the quadruple asterisk indicates P<.0001. D–E, Dot plots showing relative (D) spike- and (E) RBD-specific maternal blood (M) and cord blood (C) titers of IgG1. Wilcoxon matched-pairs signed rank test was performed to determine significance. At the right of each panel, the x-axis shows the time from the second vaccine to delivery and the y-axis shows cord blood log10 transformed titer for (D) IgG spike (purple) and (E) IgG RBD (turquoise). Correlation was determined by Spearman correlation test. PBS background subtraction was used to determine corrected optical density (OD) of 0.0. F, Neutralizing antibody titers (50% inhibitory dose) of maternal blood (M) and cord blood (C) are presented. Wilcoxon matched-pairs signed rank test was performed to determine significance.
Ig, immunoglobulin; PBS, phosphate-buffer saline; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TR, transfer ratio.
Gray et al. Coronavirus disease 2019 vaccination in pregnancy and lactation. Am J Obstet Gynecol 2021.