Figure 3.
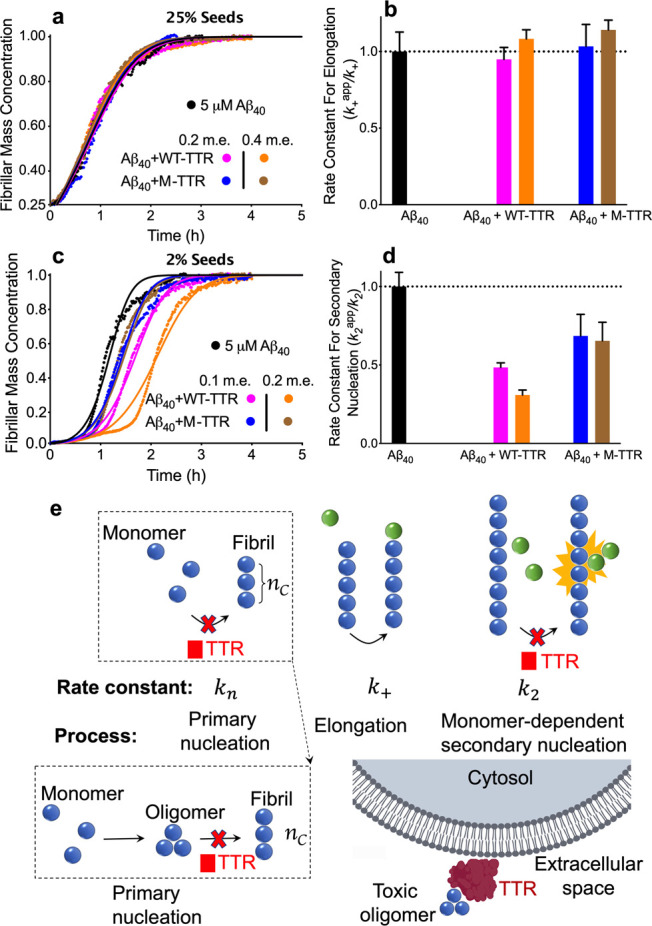
WT-TTR and M-TTR cause significant inhibition of secondary nucleation, but not elongation, in Aβ40 aggregation. (a) Kinetic profiles of a 5.0 μM Aβ40 solution with 25% of preformed seeds (m.e.) in the absence and presence of 0.2 or 0.4 m.e. of M-TTR and WT-TTR. Solid lines are fits of the reaction profiles when elongation (k+) is allowed to vary by the different TTR species. (b) Normalized rate constants for fibril elongation derived from the fitted curves in (a). Error bars are s.d. (c) Kinetic profiles of a 5.0 μM Aβ40 solution with 2% of preformed seeds (m.e.) in the absence and presence of 0.1 or 0.2 m.e. of M-TTR or WT-TTR. Solid lines represent predictions for the resulting reaction profiles when secondary nucleation (k2) is allowed to vary by the different TTR species. (d) Normalized rate constants for secondary nucleation derived from the fitted curves in (c). Error bars are s.d. (e) Scheme of the proposed protective mechanisms operated by WT-TTR and M-TTR on Aβ fibril formation and its toxicity. Top: microscopic processes of amyloid fibril formation and associated rate constants slowed down by WT-TTR and M-TTR (red crosses). Bottom right: binding of TTR molecules to Aβ oligomers and inhibition of their toxicity.
