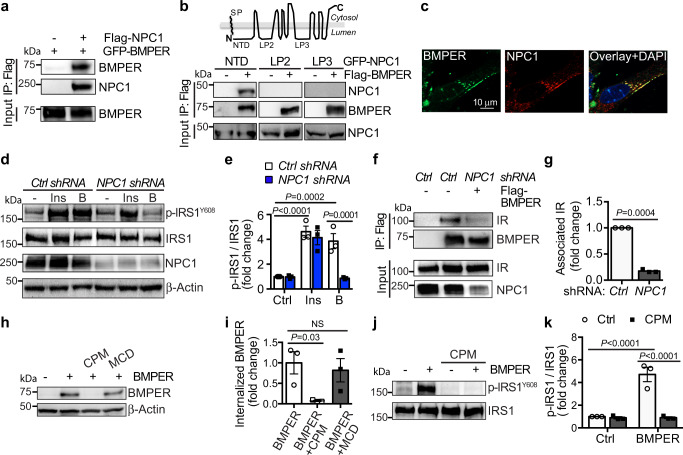
Fig. 5. BMPER promotes insulin signaling through NPC1 and endocytosis.
a Co-immunoprecipitation (IP) of GFP-tagged BMPER and flag-tagged NPC1 in HEK293 cells. b Co-immunoprecipitation of GFP-tagged NPC1 constructs containing different lumenal domains (NTD, N-terminal domain, a.a. 25-164; LP2, loop 2, a.a. 371-615; LP3, loop 3, a.a. 855-1098) and flag-tagged BMPER in HEK293 cells. SP signal peptide. c Hepatocytes were treated with flag-tagged BMPER for 30 min and staining of BMPER and endogenous NPC1 was performed. d, e Hepatocytes were transduced with NPC1 shRNA lentivirus and then treated with flag-tagged BMPER (B, 1 h) or insulin (Ins, 30 min). Western blotting was performed and band intensity was quantified (e). f, g Hepatocytes were transduced with NPC1 shRNA lentivirus and then treated with flag-tagged BMPER (B, 1 h). Membrane fractions were separated and then subjected for IP with flag antibody. The associated IR with BMPER was quantified (g). h, i Hepatocytes were treated with chlorpromazine (CPM, 50 μM) or methyl-β-cyclodextrin (MCD, 10 mM). Thirty minutes later, cells were pulsed with flag-BMPER (100 nM) for 1 h and cell lysates were used for Western blotting. Internalized BMPER was quantified in (i). j, k Hepatocytes were treated with CPM for 30 min, and then treated with BMPER for the detection of IRS1 phosphorylation. n = 3 repeated experiments (e, g, i, k). Data are presented as mean values ± SEM. NS not significant. Analysis was two-way ANOVA (for e, k) or one-way ANOVA (for i) followed by Fisher’s LSD multiple comparison test and unpaired two-tailed Student’s t test (g).