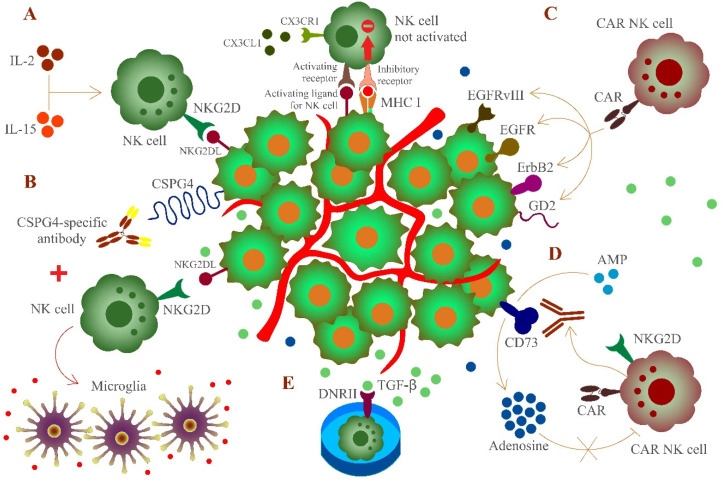
Figure 1.
Targeting of glioblastoma cells expressing major histocompatibility complex class I (MHC I) molecules by activated or engineered natural killer (NK) cells. The chemokine receptor CX3CR1 is expressed on NK cells. NK cells can follow a CX3CL1 gradient to cross the blood brain barrier to the site of glioblastoma. However, glioblastoma cells partly express MHC I molecules to impair recognition by NK cells as an immune evasion strategy. To circumvent this obstacle in NK cell-mediated immunotherapy, several procedures were developed. (A) Induced upregulation of NKG2D or cytokine (IL-2 and IL-15) -activated NK cells can overcome immune surveillance, (B) combined use of activated NK cells and monoclonal antibodies recognizing CSPG-4 leads to the recruitment of macrophages and differentiate to microglia, provoking a proinflammatory environment, (C) chimeric antigen receptor (CAR)-engineered NK cells can target epidermal growth factor receptor variant III (EGFRvIII), epidermal growth factor receptor (EGFR), ErbB2, or disialoganglioside GD2 on glioblastoma cells, (D) engineered NK cells expressing the GD2- and NKG2D-based CARs can locally release an antibody fragment which blocks the activity of CD73 and decreases adenosine concentration, which in turn is not able to inhibit NK cell function, and (E) umbilical cord blood (UCB)-derived NK cells expressing the TGF-β-dominant-negative receptor II (DNRII) that is able to consume TGF-β molecules, thereby counteracting the immune-suppressive tumor environment of glioblastoma. MHC I molecules and inhibitory receptors are not shown for all cells to improve the overview.