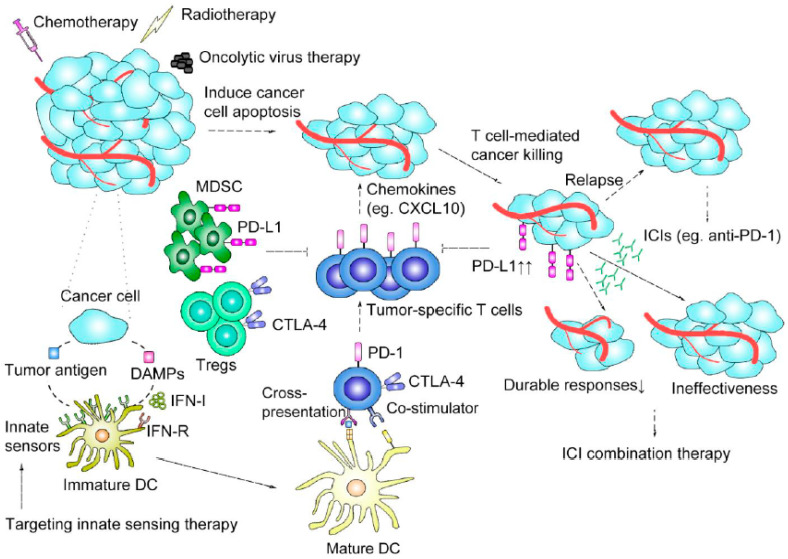
Figure 1.
Immune-based mechanisms of immune checkpoint inhibitor (ICI) therapy and combination strategy rationales. Conventional cancer therapies, including chemotherapy, radiotherapy, and targeted therapy, induce cancer cell apoptosis as well as release tumor-associated antigens and various damage-associated molecular patterns (DAMPs) to activate dendritic cells (DCs) by triggering innate immune sensing pathways and type I interferons (IFN-I) ligating IFN receptors (IFN-Rs) on DCs. The interactions between cancer cells and activated DCs promote the cross-priming and recruitment (though chemoattractants such as CXCL10) of tumor-specific T cells for additional T cell–mediated killing of tumor cells. Conventional cancer therapies can also promote immunosuppression in the tumor microenvironment TME and cause resistance to immunotherapy by mechanisms involving the upregulation of PD-L1 on tumor cells and the accumulation of myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs). ICIs can restore the function of exhausted T cells or enhance that of cytotoxic CD8+ T cells to regain antitumor responses. Regarding ICI treatment failure attributable to initial ineffectiveness or the lack of durable responses, combination treatments involving the joint application of conventional therapies or immunotherapy can enhance the specific antitumor immune response, thereby achieving better tumor control. Oncolytic virus therapy, therapies targeting innate sensors, and other immunotherapies can strengthen the antitumor immune response.