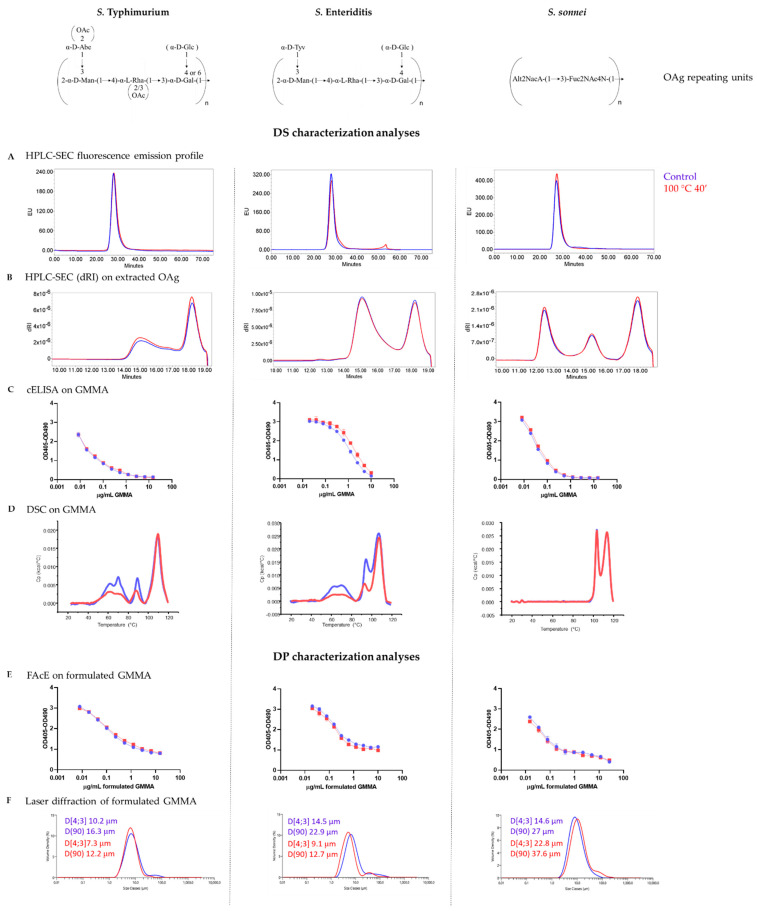
Figure 1.
Stability of S. Typhimurium, S. Enteriditis and S. sonnei GMMA, both drug substance (A–D) and drug product (E,F), under very harsh conditions (100 °C for 40 min). No change in HPLC-SEC fluorescence emission profiles of stressed GMMA (A) and in OAg size populations distribution (B). No differences in anti-OAg specific mAb recognition for all three different types of GMMA, both drug substance (C) and drug product (E). Evidence of protein denaturation occurring in iNTS GMMA (D). No changes detected in terms of particle size (F) when GMMA were stressed after formulation on Alhydrogel. Blu lines refer to control GMMA, red lines to stressed GMMA.