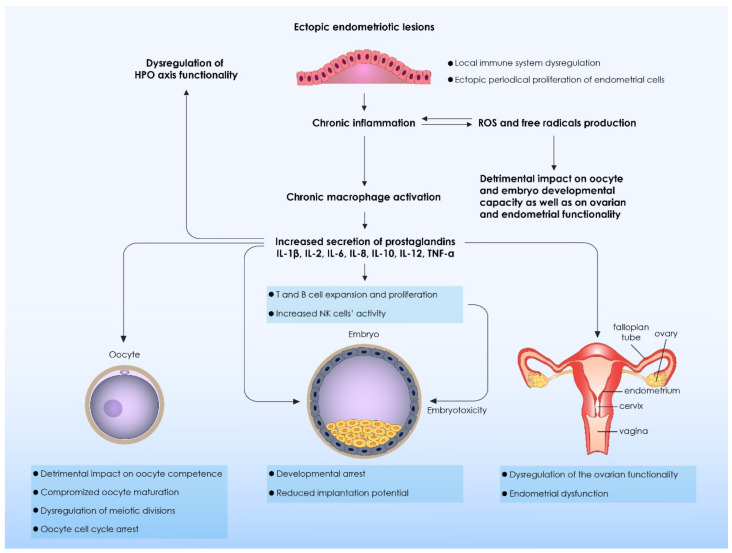
Figure 2.
The impact of disruption of the immune system balance on oocyte quality and embryo developmental capacity. Immune system dysregulation is observed in the areas where ectopic endometriotic lesions are localized. As a results a periodical proliferation and activation of the ectopic endometrial cells is observed. High proliferation levels lead to an increased production and secretion of ROS from the ectopic endometriotic lesions. The increased ROS and free radicals production leads to the stimulation of proinflammatory phenomena and as a result a chronic inflammation is established. Endometrial cells promote inflammation and therefore a chronic activation of macrophages in the peritoneal fluid. Activated macrophages secrete a high amount of cytokines and prostaglandins. From one hand, secreted cytokines induce lymphocytes’ and natural killer cells’ (NK) activation and proliferation. These activated immune cells directly affect embryo development inducing embryotoxic phenomena and leading to embryo developmental arrest. Affected embryos are characterized by a significantly reduced implantation potential. On the other hand, cytokines and prostaglandins directly affect oocyte and embryos, jeopardizing oocyte competence and oocyte maturation. As a result, inflammation leads to dysregulation of meiotic divisions, inducing oocyte cell cycle arrest. Additionally, cytokines directly affect HPO axis regulation as well as ovarian and endometrial functionality affecting the ovulation process and endometrial receptivity, respectively. ROS: Reactive Oxygen Species; IL: Interleukin; TNF-a: Tumor Necrosis Factor a; HPO axis: Hypothalamus Pituitary Ovarian axis.