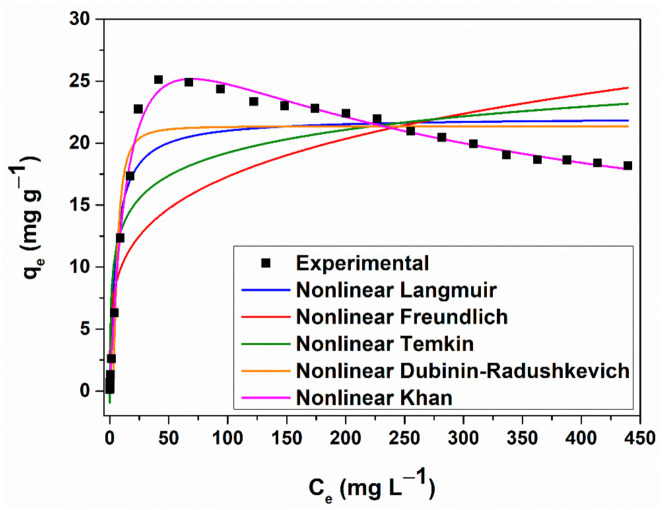
Figure 8.
Nonlinear forms of the equilibrium isotherms obtained for CV sorption onto MNP@PAAA-FA Considering both the lowest standard deviation value and the highest coefficient of determination, it can be stated that Khan isotherm model fits the best CV adsorption process. Thus, the αK value (1.38) is around 1, indicates that the CV adsorption process approaches the Langmuir isotherm at lower concentrations, while at higher values, this model reduces to the Freundlich isotherm.