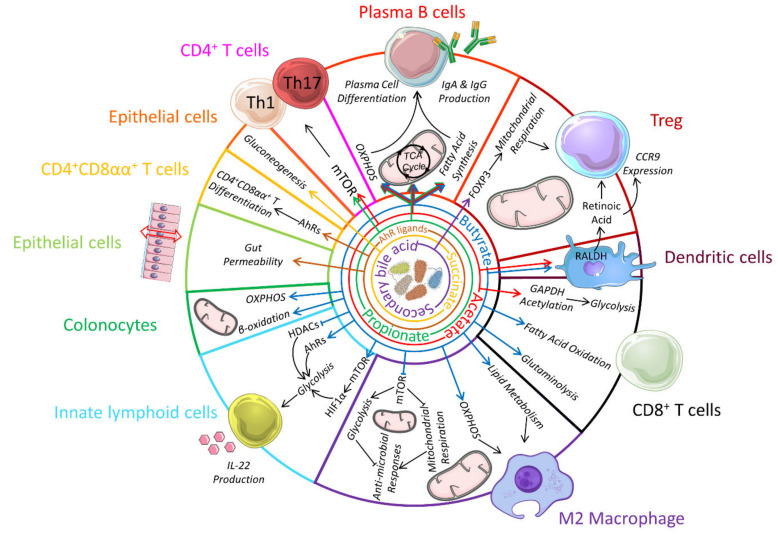
Figure 2.
Impact of bacterial-derived metabolites on host immunometabolism and gut immune cell function. Butyrate can be oxidised by colonocytes to generate energy via oxidative phosphorylation (OXPHOS), or to promote metabolic rewiring of immune cells such as M2 macrophages and CD8+ memory T cells towards fatty acid oxidation and OXPHOS. Butyrate also inhibits histone deacetylase (HDAC), and upregulate aryl hydrocarbon (AhR) receptor and hypoxia-inducible factor 1-alpha (HIF-1α) expression to promote glycolysis in innate immune cells to drive their production of IL-22. Acetate and propionate induces Th1/Th17 differentiation by activating the mechanistic target of rapamycin (mTOR), while acetate and butyrate promote regulatory T cell (Treg) differentiation by enhancing retinaldehyde dehydrogenase (RALDH) enzyme activity in tolerogenic dendritic cells to produce retinoic acid. Retinoic acid also induce the gut homing receptor C-C chemokine receptor type 9 (CCR9) in Treg. Acetate also enhances memory CD8+ T cell effector function by enhancing glyceraldehyde 3-phosphate dehydrogenase (GAPDH) enzyme activity to drive glycolysis. Butyrate, acetate and propionate can promote plasma B cell differentiation and antibody production by activating OXPHOS and fatty acid synthesis, and to promote immunoglobulin A (IgA) and immunoglobulin G (IgG) production. In the gut, succinate act as a substrate for gluconeogenesis in epithelial cells, and may also potentially serve as a pro-inflammatory molecule (not shown). Secondary bile acids, such as 3-oxo lithocholic acid, can directly induce the differentiation RORγt Treg in the colon while AhR ligands can decrease gut permeability, as well as drive the differentiation of CD4+CD8αα+ T cells.