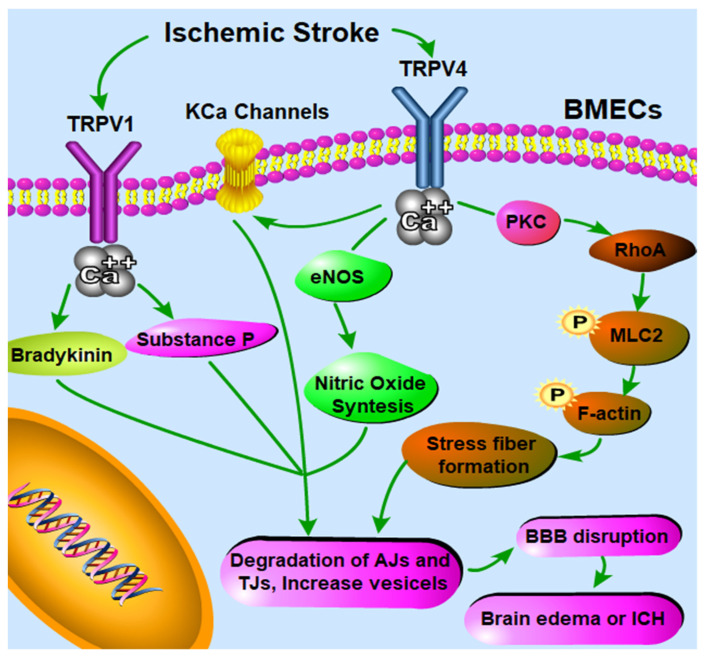
Figure 1.
Schematic illustration describing the possible involvement of Transient Receptor Potential Vanilloid (TRPV) in the context of blood brain barrier (BBB) function under ischemic stroke according to current knowledge. TRPV1 antagonism can block bradykinin and Substance P release mediated BBB disruption during cerebral ischemia [62]. The mechanisms underlying TRPV4-mediated BBB permeability modulation are complex. Firstly, Ca2+ influx, which is elicited by activation of endothelial TRPV4 channels, can further activate intermediate and small conductance Ca2+-sensitive K+ (KCa) channels [114], while KCa channels have been well known to be able to induce BBB opening [115]. Secondly, increased intracellular Ca2+ via TRPV4 activation can also significantly induce the endothelial nitric oxide synthase (eNOS) phosphorylation [105], and activated eNOS produces more nitric oxide (NO), further activating soluble guanylyl cyclase to produce more cyclic guanosine monophosphate (cGMP); high concentrations of cGMP lead to an increase in the number of pinocytic vesicles containing caveolin-1 and caveolin-2 and BBB permeability [116]. Thirdly, another possible mechanism involved in TRPV4 activation mediated BBB disruption is the formation of stress fibers through the PKCα/RhoA/MLC2 pathway activation. PKCα activation has been involved in TRPV4-regulated endothelial cell permeability alteration [117]. After activation, PKCα can directly interact with RhoA, leading to the rearrangement of the cytoskeleton [118,119]. RhoA further binds to Rho-kinase, which can strongly induce the phosphorylation of myosin light chain 2 (MLC2) and the formation of actin stress fibers [120], thus leading to BBB disruption [121]. Therefore, TRPV4 antagonism is able to inhibit actin stress fibers formation in ischemia, thus preserving/rescuing the integrity of the BBB [101].