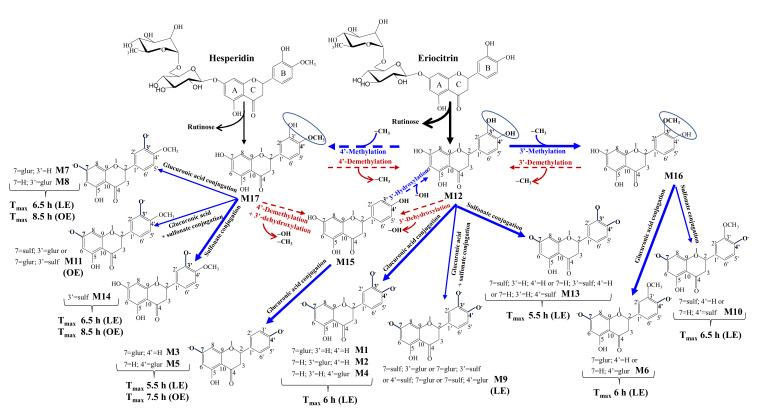
Figure 4.
Main metabolic transformations of hesperidin and eriocitrin to their corresponding phase-II conjugates. The identification and quantification of metabolites (M) can be found in Table 3 and Table 4, respectively. The pharmacokinetic values can be found in Table 5. Black lines, microbial metabolism; blue lines, phase-II metabolism; red lines, phase-I or microbial metabolism; dashed lines, proposed new metabolic steps in the human metabolism of hesperidin and eriocitrin. The thickness of the lines represents the most favored pathways. LE, lemon extract; OE, orange extract (hesperidin); glur, glucuronic acid; sulf, sulfonic acid. The formation of eriodictyol (M12) from hesperetin mainly occurs via 4′-demethylation of hesperetin (M17) and the formation of naringenin (M15), after 4′-demethylation plus 3′-dehydroxylation. Besides, M12 could also be formed after CYP-catalyzed 3′-hydroxylation of M15 (Figure 4). These steps were supported by the detection of eriodictyol and naringenin conjugates after hesperidin intake in plasma and urine. However, no homoeriodictyol or derived metabolites (M16, M6, M10) were observed after hesperidin intake, which indicates that 4′-methylation to yield back hesperetin was favored instead of the subsequent 3′-methylation of eriodictyol to yield homoeriodictyol after consuming hesperidin (Figure 4).