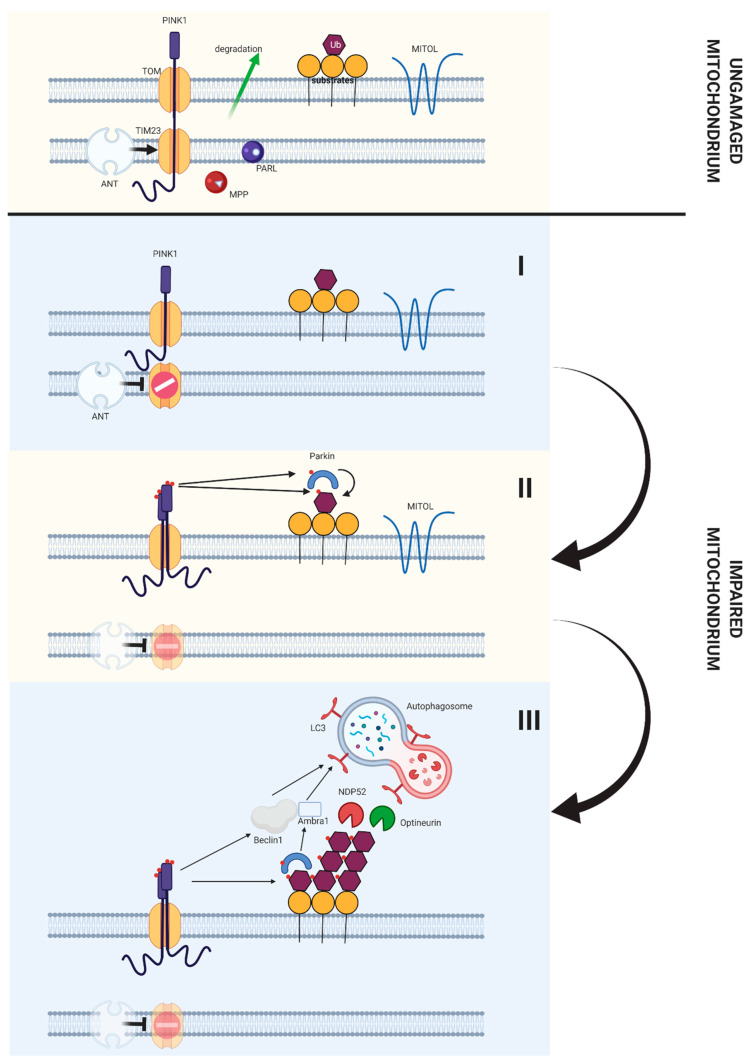
Figure 6.
Role of the PINK1/Parkin pathway in induction of autophagy both in stem cells and differentiated cells. In a properly functioning mitochondria PINK1 is transported through the TOM/TIM23 translocase complex to the internal mitochondrial membrane. It is then disintegrated (due to the activity of two proteases, PARL and MPP), transported to cytosol and degraded. When mitochondria do not functioning properly (which manifests itself as a dysfunction of mitochondrial membrane potential), ANT inhibits the transport of PINK1 through the TIM23 translocase. As a result of this phenomenon PINK1 accumulates on the external mitochondrial membrane. Accumulation of PINK1 on the external mitochondrial membrane results in formation of complex consisting of PINK1 and TOM translocase, which results in autophosphorylation of PINK1 and its activation. The activated PINK1 phosphorises the ubiquitinated substrates located on the external mitochondrial membrane, which makes it possible to connect Parkin and its phosphorylation. Phosphorylated Parkin, in turn, contributes to the ubiquitination of subsequent proteins on the external mitochondrial membrane. The ubiquitinated proteins, in the other hand, are able to phosphorylate subsequent PINK1 molecules, thus creating a feedback loop, so that a malfunctioning mitochondrion is covered with phosphoubiquitin. Then mitophagic adapters Optineurin and NDP52 bind phosphoubiquitin chains. These proteins further bind the LC3 protein, which allows the initiation of mitophagy. At the same time PINK1 interacts with Beclin-1, Parkin and Ambra proteins, which contributes to the formation of autofagosome around the damaged mitochondria [182].