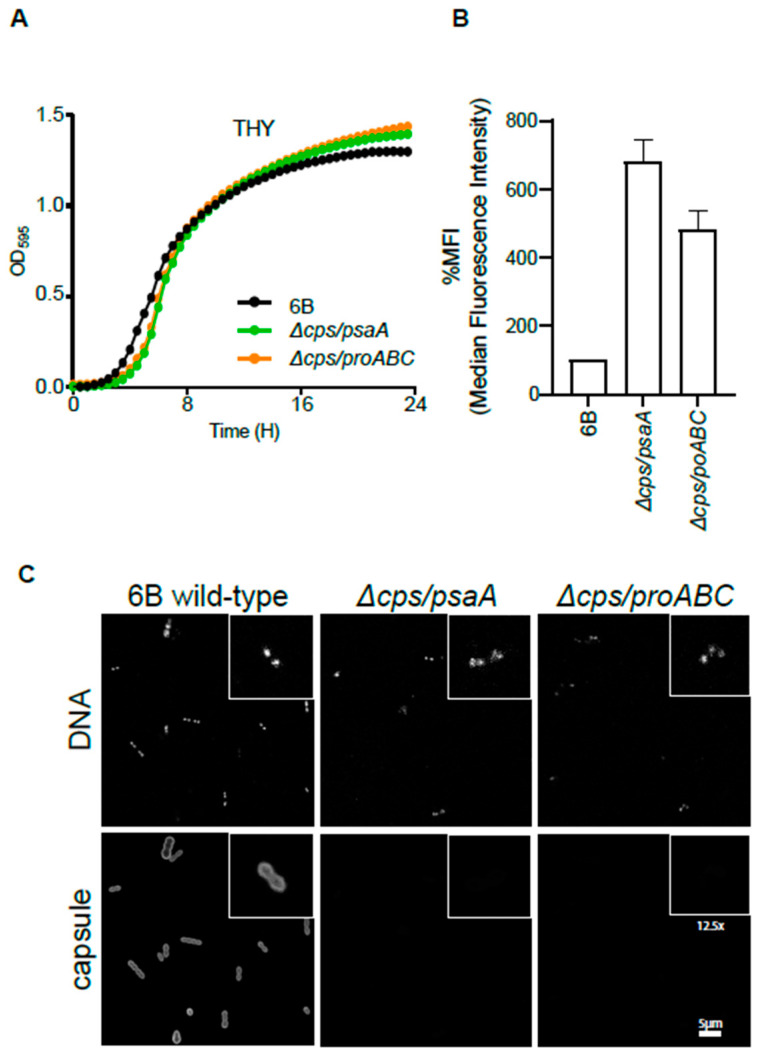
Figure 1.
Phenotypic characterization of double unencapsulated mutants ∆cps/psaA and ∆cps/proABC. (A) Growth of wild type and unencapsulated strains ∆cps/psaA and ∆cps/proABC in THY assessed by measuring OD595 for a 24 h period. (B) Effect of the S. pneumoniae capsule on C3b deposition. Median fluorescence intensity (MFI) of C3b deposition measured using flow cytometry on the wild type 6B and unencapsulated mutants when incubated in 25% human serum. Error bars represent SDs and asterisks represent the differences between wild type and the unencapsulated mutant strain. For both mutant strains, p is < 0.0001 (one way ANOVA) compared to the wild type strain. (C) Fluorescent microscopy of wild type and unencapsulated double mutant strains following incubation with 4′,6-diamidino-2-phenylindole (DAPI) (binds to DNA to identify bacterial cells, top panels) or pneumococcal antiserum labelled with Alexa fluor 546 (recognizes serotype 6 capsule, bottom panels). The scale bar (bottom right) represents 5 µm and the inserts show a 12.5 higher magnification of selected bacteria.