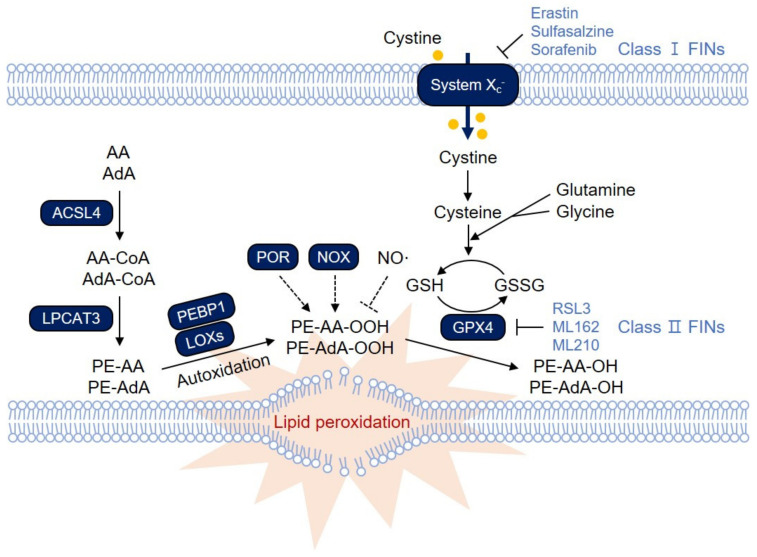
Figure 1.
The ferroptosis signaling pathway. Polyunsaturated fatty acids (PUFAs) in membrane phospholipids undergo lipid peroxidation, which directly destroys the cellular membrane, thereby causing necrotic cell death via ferroptosis. Glutathione Peroxidase 4 (GPX4) reduces lipid peroxide to lipid alcohol by oxidizing glutathione (GSH), thereby protecting cells from ferroptosis under normal conditions. Inactivation of GPX4 or depletion of GSH therefore leads to massive lipid peroxidation and induces ferroptosis. Ferroptosis-inducing compounds (FINs) are categorized into two main groups: those that inhibit system xc−, thereby depleting GSH levels (class I FINs), and those that directly inhibit GPX4 (class II FINs). Among various membrane phospholipids, arachidonic acid (AA)- and adrenic acid (AdA)-containing phosphatidylethanolamine (PE) and phosphatidylcholine (PC) are the primary targets for lipid peroxidation. Acyl-CoA synthetase long-chain family member 4 (ACSL4) links free PUFAs to CoA, generating fatty acyl-CoA esters, which are eventually incorporated into PC/PE by lysophosphatidylcholine acyltransferase 3 (LPCAT3). PE-AA and PE-AdA can be oxidized by lipoxygenases (LOXs). LOX might require phosphatidylethanolamine-binding protein 1 (PEBP1) to induce lipid peroxidation on the membrane. In addition, other oxygenases, such as NADPH oxidases (NOXs) and cytochrome P450 oxidoreductase (POR), are known to contribute to lipid peroxidation. Lipid peroxidation is also mediated by nonenzymatic autoxidation, which is suggested to be the ultimate driver of ferroptotic cell death. In contrast, NO• reacts with lipid peroxyradicals, thereby attenuating lipid peroxidation and ferroptosis.