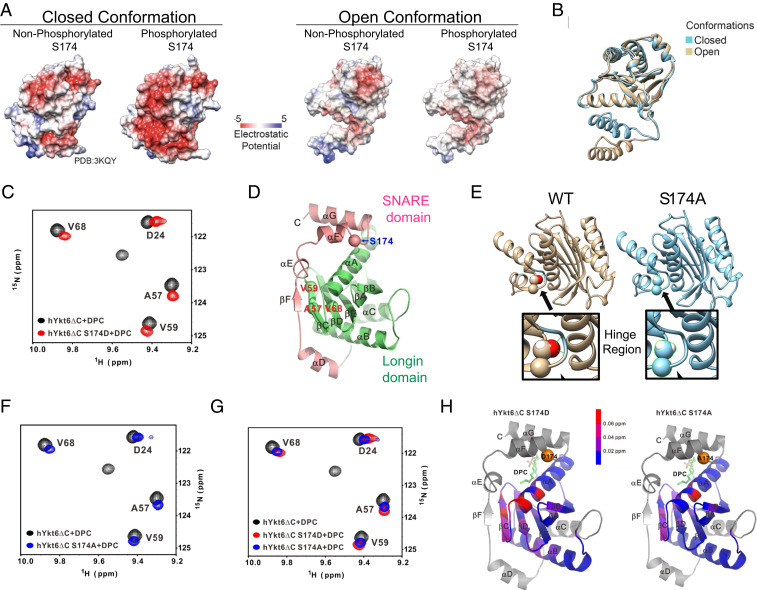
Fig. 2.
Calcineurin-sensitive evolutionarily conserved phosphorylation site S174 in Ykt6 SNARE domain regulates its conformation. (A) Electrostatic potential computed and projected at the surface representation of both open and closed conformation in the presence and absence of phosphorylation at the evolutionarily conserved site S174. (B) Ykt6 secondary structure depicting the open (brown) and closed (blue) conformation. (C) 15N-1H TROSY of 0.4 mM 25 kDa Ykt6 at 600 MHz. WT Ykt6 (black) versus S174D (red) in the presence of DPC. (D) Ykt6 crystal structure. Longin domain (green) and the SNARE domain (pink); in red are the amino acids where a chemical shift was detected and in blue is the S174 phosphorylation site. (E) Structural model showing the critical positioning of S174 in the hinge loop and how the alanine replacement loosens the interaction of between the SNARE and the longin domains. The hinge region is enlarged for both the WT and phosphoablative S174A Ykt6. (F) 15N-1H TROSY of 0.4 mM 25 kDa Ykt6 at 600 MHz. WT Ykt6 (black) and S174A (blue) in the presence of DPC. (G) 15N-1H TROSY of 0.4 mM 25 kDa Ykt6 at 600 MHz. WT Ykt6 (black) and phosphomimetic S174D (red) and phosphoablative S174A (blue) in the presence of DPC (J). (H) Ykt6 crystal structure denoting chemical shifts detected throughout the protein; below 0.02 ppm is not significant.