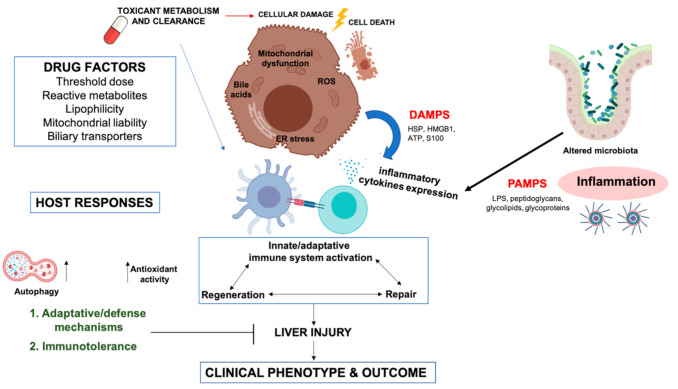
Figure 1.
Cellular and molecular mechanisms involved in idiosyncratic drug-induced liver injury. Two key players in DILI, drug and host factors may interact in a multi-faceted manner at different functional pathways and determine individual susceptibility, clinical phenotype, and outcome. The hepatocyte damage caused by the action of drugs induce a complex, multivariant host response. First, cellular damage (oxidative stress, mitochondrial dysfunction, endoplasmic reticulum (ER) stress, and bile salt export pump (BSEP) inhibition, between others) can lead to cell death, provoking cell swelling and eventually rupture of the cell membrane, with the release of intracellular content, including damage-associated molecular patterns (damage-associated molecular patterns (DAMPs), such as high mobility group box protein 1 (HMGB1), heat shock proteins (HSP), ATP, S100 proteins, etc.) which stimulate a strong inflammatory/immune response. Inflammasome has also a very important role in development of liver injury, inducing cytokines secretion to attract and activate macrophages and neutrophils. Moreover, drugs can also alter intestinal microbiota (dysbiosis), and increase intestinal permeability, releasing bacterial products (Pathogen-associated molecular patterns, [PAMPs]) into the bloodstream. PAMPs (bacterial lipopolysaccharides (LPS), endotoxins, flagellin, etc.) act as costimulatory signals for the innate immune system activation. DAMPs and PAMPs are able to bind to TLR of the innate immune cells potentiating the immune response, cytokine release, and immune cell recruitment. Furthermore, drugs can form drug-endogenous proteins adducts that can act as neoantigens. Neoantigens presentation on specific HLA molecules could cause an adaptive immune response. Some HLA polymorphisms favor the presentation of drug-adducted neoantigens. Thus, individuals carrying the HLA variant are more susceptible to develop an adaptive immune response, typically leading to a T cell response directed at hepatocytes and usually involving cytotoxic CD8 T cells that target the peptide drug exposed on MHC class I molecules on the hepatocytes. Cellular damage also induces host adaptive and defense mechanisms, such as autophagy, antioxidant response and tissue repair. Moreover, because of its biological role with constant exposure to foreign antigens, the liver has a strong natural predisposition towards immune tolerance. This tolerance prevents a substantial immune response in the presence of the chemical insult, causing, at most, a mild liver injury that resolves spontaneously despite continued drug intake (i.e., adaptation). Clinically relevant liver injury is believed to result from a breakdown in hepatic immune tolerance. Concomitant inflammation can change the cytokine environment in favor of an immune response. Host factors such as age, gender, genetic factors, lifestyles, disease conditions, and co-medications are involved in the susceptibility of significant liver damage.