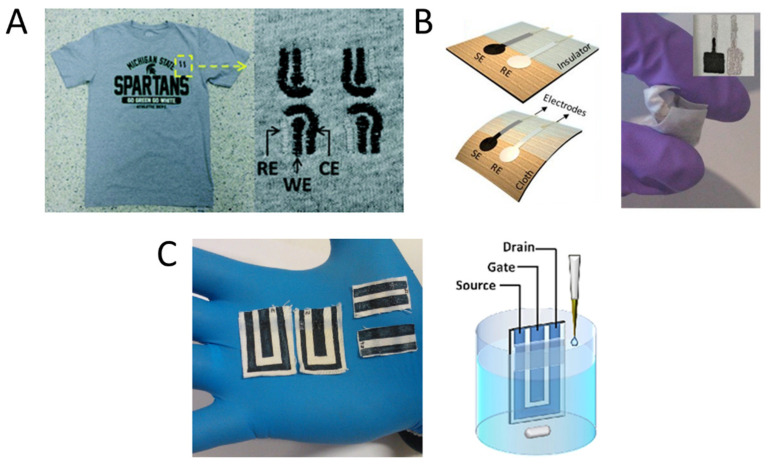
Figure 1.
Examples of different kinds of electrochemical textile sensors. (A) Example of a textile amperometric sensor [17]. An amperometric sensor exploits the current associated to a redox reaction occurring at a working electrode (WE) as the analytical signal. The WE potential is usually fixed with respect to a reference electrode (RE) and both electrodes must be immersed in the same solution. The most rigorous measurements are performed in a three-electrode cell endowed with a counter electrode (CE). Reproduced from Ref. [16] with permission from the Royal Society of Chemistry. (B) Example of potentiometric sensors [16]. A potentiometric sensor is based on the measurement of the potential difference between the indicator electrode (SE) and a reference electrode (RE) that must be immersed in the solution under investigation. The Nernst equation describes the dependence of the potential on the logarithm of the analyte concentration. The signal stems from redox reactions involving the analyte or a membrane that selectively allows its adsorption or passage. (C) Example of an organic electrochemical transistor (OECT) printed on a cotton fabric [20]. OECTs are constituted of a channel made of a conductive polymer and a gate electrode. Both elements must be immersed in an electrolytic solution. When a voltage is applied to the gate electrode, electrochemical reactions take place at the channel that vary the charge concentration in the conductive polymer. Any substance that changes the current flow in the channel can be detected by these devices.