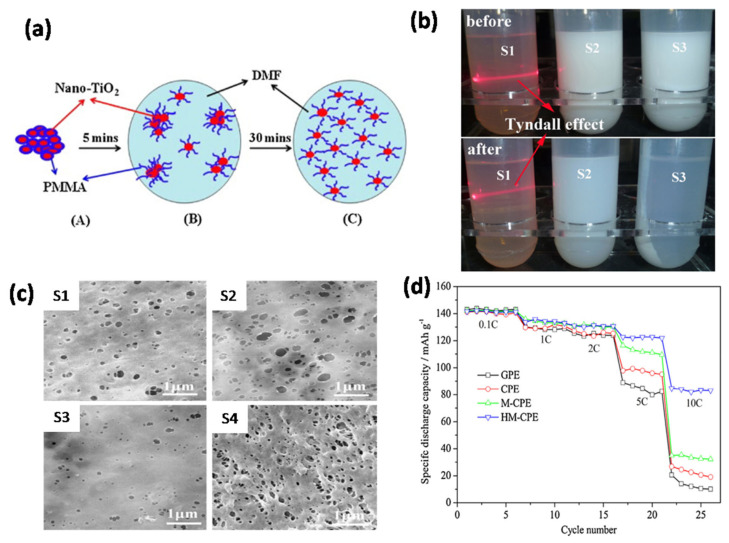
Figure 6.
(a) Schematic of nano-TiO2- poly(methyl methacrylate) (PMMA) in N,N-dimethylformamide (DMF) (A: mixture of nano-TiO2 and PMMA, B: tethered PMMA on Nano-TiO2, C: self-assembly of PMMA-tethered Nano-TiO2) and (b) photographs of the dispersed NPs in DMF before (top panel) and after (bottom panel) 10 min centrifugation at 10,000 rpm. The same TiO2 content (5 wt%) was used in each sample: (S1) highly dispersed nano-TiO2-PMMA, (S2) nano-TiO2-PMMA, and (S3) pristine nano-TiO2. (c) SEM images of the GPEs: (S1) pristine PVDF-HFP (“GPE”), (S2) nano-TiO2/PVDF-HFP (“CPE”), (S3) nano-TiO2-PMMA/PVDF-HFP (“M-CPE”), and (S4) highly dispersed nano-TiO2-PMMA/PVDF-HFP (“HM-CPE”). (d) The rate capabilities of the different electrolytes shown in (c). Reprinted with permission from Chen et al. [85]. Copyright 2013 Elsevier Ltd.