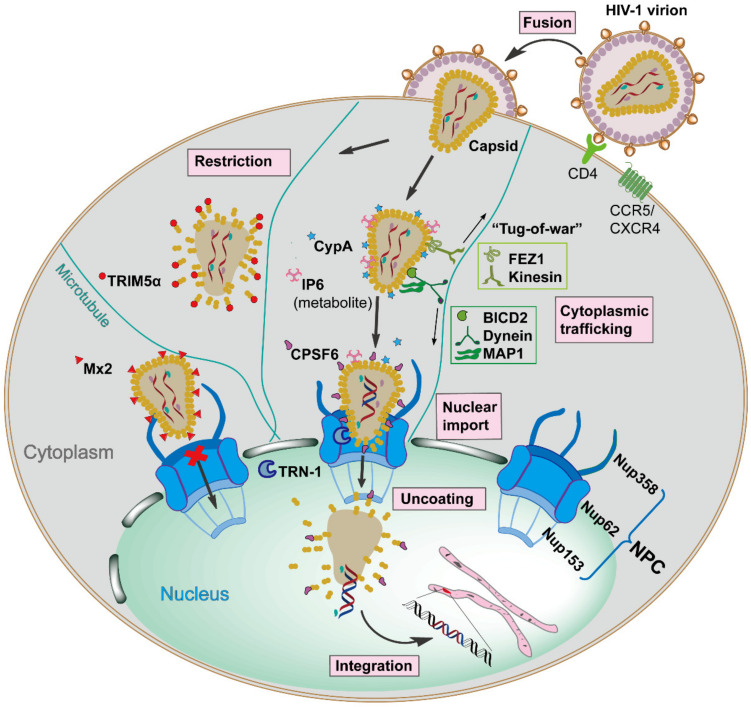
Figure 1.
Schematic overview of the early stages of Human Immunodeficiency Virus type 1 (HIV-1) replication, which is spatiotemporally regulated by diverse capsid-interacting host factors. The HIV-1 virion binds to the CD4 receptor and the CXCR4 and/or CCR5 coreceptors via the viral envelope glycoproteins, resulting in fusion with the target cell, which releases capsid into the cellular cytoplasm. The capsid traffics towards the nucleus along the microtubule network by employing opposing adaptor and motor proteins such as FEZ1, kinesin, BICD2, dynein, and MAP1, a process that may result in a bi-directional “tug-of-war”. The host cellular protein CypA and metabolite IP6 bind to capsid and maintain core stability during cytoplasmic trafficking. The host innate immune system can respond to capsid, resulting in interferon (IFN) expression and inducing the IFN-induced viral restriction factors TRIM5α and Mx2. TRIM5α binds to the capsid causing premature uncoating, while Mx2 binds to the capsid impeding the nuclear import. When capsid arrives at the nuclear envelope, it presumably binds Nup358, and other factors, to promote import into the nucleus via the nuclear pore complex as an intact or nearly intact capsid. The host factors CPSF6, Nup153, and TRN-1 also appear to facilitate capsid nuclear import via direct capsid interactions. Reverse transcription is completed in the nucleus, followed by complete uncoating of the capsid and integration of the viral DNA into the host genome. All processes are detailed in the text.