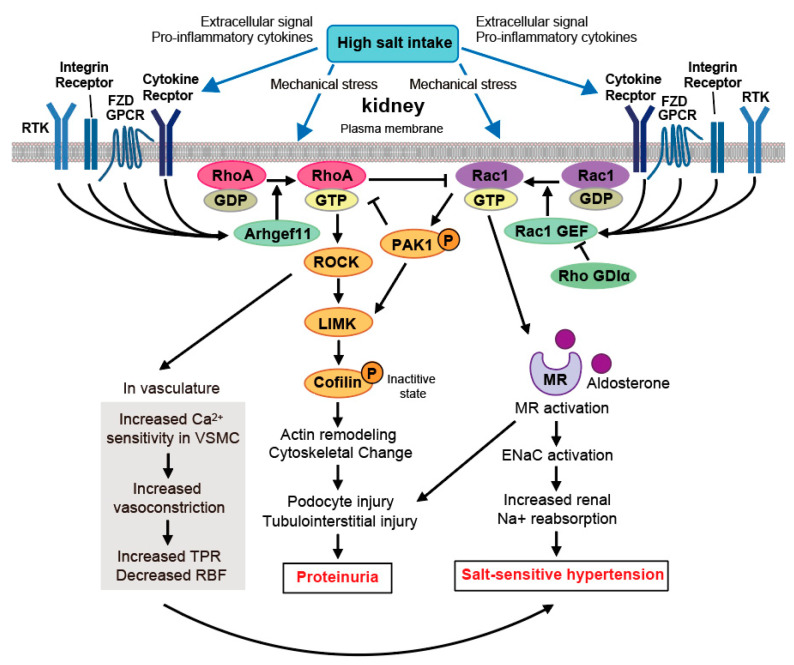
Figure 2.
Cross talk between RhoA and Rac1 in salt-sensitive hypertension and renal injury in the kidney of Dahl salt-sensitive (DS) rats. In DS rats, a high-salt diet causes salt-sensitive hypertension and exacerbates renal damage, which is closely related to the activation of RhoA and Rac1 in the kidney. Various extracellular stimuli activate Rho GTPases via their receptors such as G-protein coupled receptors (GPCRs) [115], receptors of the tyrosine kinase (RTKs) [116], cytokine receptors [117], and integrin receptors [118] as well as Wnt and Notch signaling [119,120] by activating specific guanine nucleotide exchange factors (GEFs) [121]. Mechanical stress also stimulates RhoA and Rac1 [122]. It is known that salt intake increases the production of cytokines such as IL-1β and IL-17 in the blood, resulting in salt-sensitive hypertension [123] and that cytokines activate Rac1 [117]. In addition, Wnt5a is produced by macrophages and adipocytes via paracrine and autocrine upon external stimuli and further promotes cytokine production and secretion [124]. While Wnt5a is involved in enhancing inflammation, it activates RhoA [13,125]. These findings suggest that salt intake stimulates cytokine and Wnt protein production from macrophages and tissues and activates Rac1 and Rho, although the detailed mechanism of Rac1 and RhoA activation by high-salt diet is still unknown. In the kidneys of high-salt-fed DS rats, RhoA is activated by Arhgef11, which is activated by salt intake, and ROCK and LIM-kinase (LIMK) are sequentially activated, and cofilin activity is suppressed, resulting in actin polymerization and remodeling in glomerulus tubules and interstitium leading to marked proteinuria and tubulointerstitial injury. In the vasculature, activated ROCK increases vasoconstriction through increased Ca2+ sensitivity in VSMCs, increases total peripheral resistance (TPR), and decreases renal blood flow (RBF), thereby contributing to the development of salt-sensitive hypertension. On the other hand, salt intake activates Rac1 by Rac1-GEF, which synergistically activates mineralocorticoid receptors (MR) with aldosterone and increases sodium reabsorption via epithelial sodium channel (ENaC) in the renal collecting duct, resulting in salt-sensitive hypertension with glomerular and tubulointerstitial injury. Rac1 activates downstream p21-activated kinase 1 (PAK1), but PAK inhibits RhoA, and RhoA inhibits Rac1. Since PAK activates LIMK, there is LIMK-mediated crosstalk between RhoA and Rac1 in the DS kidney, and both are involved in the development of salt-induced renal injury.